Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii served as key components of fecal microbiota transplantation to alleviate colitis
- PMID: 38502145
- PMCID: PMC11376976
- DOI: 10.1152/ajpgi.00303.2023
Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii served as key components of fecal microbiota transplantation to alleviate colitis
Abstract
Fecal microbiota transplantation (FMT) is a promising therapy for inflammatory bowel disease (IBD) via rectifying gut microbiota. The aim of this study was to identify a mechanism of how specific bacteria-associated immune response contributes to alleviated colitis. Forty donors were divided into high (donor H) and low (donor L) groups according to the diversity and the abundance of Bacteroides and Faecalibacterium by 16S rRNA sequencing. FMT was performed on dextran sulfate sodium (DSS)-induced colitis in mice. Mice with colitis showed significant improvement in intestinal injury and immune imbalance after FMT with group donor H (P < 0.05). Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii were identified as targeted strains in donor feces by real-time PCR and droplet digital PCR. Mice with colitis were treated with mono- or dual-bacterial gavage therapy. Dual-bacterial therapy significantly ameliorated intestinal injury compared with mono-bacterial therapy (P < 0.05). Dual-bacterial therapy increased the M2/M1 macrophage polarization and improved the Th17/Treg imbalance and elevated IL-10 production by Tregs compared with the DSS group (P < 0.05). Metabolomics showed increased abundance of lecithin in the glycerophospholipid metabolism pathway. In conclusion, B. thetaiotaomicron and F. prausnitzii, as the key bacteria in donor feces, alleviate colitis in mice. The mechanism may involve increasing lecithin and regulating IL-10 production of intestinal Tregs.NEW & NOTEWORTHY We demonstrate that donors with high abundance of Bacteroides and Faecalibacterium ameliorate dextran sulfate sodium (DSS)-induced colitis in mice by fecal microbiota transplantation (FMT). The combination therapy of Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii is superior to mono-bacterial therapy in ameliorating colitis in mice, of which mechanism may involve promoting lecithin and inducing IL-10 production of intestinal Tregs.
Keywords: Bacteroides thetaiotaomicron; Faecalibacterium prausnitzii; fecal microbiota transplantation; gut microbiota; inflammatory bowel disease.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


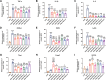
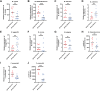

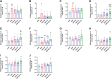

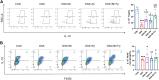

Similar articles
-
Faecalibacterium prausnitzii enhances intestinal IgA response by host-microbe derived inecalcitol in colitis.BMC Med. 2025 Jul 15;23(1):425. doi: 10.1186/s12916-025-04260-2. BMC Med. 2025. PMID: 40660288 Free PMC article.
-
Gut microbial and metabolomics profiles reveal the potential mechanism of fecal microbiota transplantation in modulating the progression of colitis-associated colorectal cancer in mice.J Transl Med. 2024 Nov 15;22(1):1028. doi: 10.1186/s12967-024-05786-4. J Transl Med. 2024. PMID: 39548468 Free PMC article.
-
Faecalibacterium prausnitzii, Bacteroides faecis and Roseburia intestinalis attenuate clinical symptoms of experimental colitis by regulating Treg/Th17 cell balance and intestinal barrier integrity.Biomed Pharmacother. 2023 Nov;167:115568. doi: 10.1016/j.biopha.2023.115568. Epub 2023 Oct 2. Biomed Pharmacother. 2023. PMID: 37793274
-
[How close is fecal microbiota transplantation to moving to precision medicine?].Zhonghua Wei Chang Wai Ke Za Zhi. 2025 Mar 25;28(3):254-260. doi: 10.3760/cma.j.cn441530-20241220-00415. Zhonghua Wei Chang Wai Ke Za Zhi. 2025. PMID: 40123396 Review. Chinese.
-
Changes in the fecal microbiota of breast cancer patients based on 16S rRNA gene sequencing: a systematic review and meta-analysis.Clin Transl Oncol. 2024 Jun;26(6):1480-1496. doi: 10.1007/s12094-023-03373-5. Epub 2024 Jan 13. Clin Transl Oncol. 2024. PMID: 38217684
Cited by
-
Uncovering de novo polyamine biosynthesis in the gut microbiome and its alteration in inflammatory bowel disease.Gut Microbes. 2025 Dec;17(1):2464225. doi: 10.1080/19490976.2025.2464225. Epub 2025 Feb 9. Gut Microbes. 2025. PMID: 39924644 Free PMC article.
-
Real-world of Limosilactobacillus reuteri in mitigation of acute experimental colitis.J Nanobiotechnology. 2025 Jan 31;23(1):65. doi: 10.1186/s12951-025-03158-8. J Nanobiotechnology. 2025. PMID: 39891249 Free PMC article.
-
Oncolytic Virotherapies and Adjuvant Gut Microbiome Therapeutics to Enhance Efficacy Against Malignant Gliomas.Viruses. 2024 Nov 14;16(11):1775. doi: 10.3390/v16111775. Viruses. 2024. PMID: 39599889 Free PMC article. Review.
-
The metabolites of gut microbiota: their role in ferroptosis in inflammatory bowel disease.Eur J Med Res. 2025 Apr 7;30(1):248. doi: 10.1186/s40001-025-02524-4. Eur J Med Res. 2025. PMID: 40189555 Free PMC article. Review.
-
Multifaceted Role of Microbiota-Derived Indole-3-Acetic Acid in Human Diseases and Its Potential Clinical Application.FASEB J. 2025 Jun 15;39(11):e70574. doi: 10.1096/fj.202500295R. FASEB J. 2025. PMID: 40415505 Free PMC article. Review.
References
-
- Ihekweazu FD, Engevik MA, Ruan W, Shi Z, Fultz R, Engevik KA, Chang-Graham AL, Freeborn J, Park ES, Venable S, Horvath TD, Haidacher SJ, Haag AM, Goodwin A, Schady DA, Hyser JM, Spinler JK, Liu Y, Versalovic J. Bacteroides ovatus promotes IL-22 production and reduces trinitrobenzene sulfonic acid-driven colonic inflammation. Am J Pathol 191: 704–719, 2021. doi:10.1016/j.ajpath.2021.01.009. - DOI - PMC - PubMed
-
- Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, Vatanen T, Hall AB, Mallick H, McIver LJ, Sauk JS, Wilson RG, Stevens BW, Scott JM, Pierce K, Deik AA, Bullock K, Imhann F, Porter JA, Zhernakova A, Fu J, Weersma RK, Wijmenga C, Clish CB, Vlamakis H, Huttenhower C, Xavier RJ. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 4: 293–305, 2019. [Erratum in Nat Microbiol 4: 898, 2019]. doi:10.1038/s41564-018-0306-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

