Drug-regulated CD33-targeted CAR T cells control AML using clinically optimized rapamycin dosing
- PMID: 38502193
- PMCID: PMC11060733
- DOI: 10.1172/JCI162593
Drug-regulated CD33-targeted CAR T cells control AML using clinically optimized rapamycin dosing
Abstract
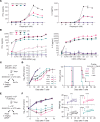
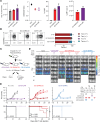
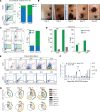
Chimeric antigen receptor (CAR) designs that incorporate pharmacologic control are desirable; however, designs suitable for clinical translation are needed. We designed a fully human, rapamycin-regulated drug product for targeting CD33+ tumors called dimerizaing agent-regulated immunoreceptor complex (DARIC33). T cell products demonstrated target-specific and rapamycin-dependent cytokine release, transcriptional responses, cytotoxicity, and in vivo antileukemic activity in the presence of as little as 1 nM rapamycin. Rapamycin withdrawal paused DARIC33-stimulated T cell effector functions, which were restored following reexposure to rapamycin, demonstrating reversible effector function control. While rapamycin-regulated DARIC33 T cells were highly sensitive to target antigen, CD34+ stem cell colony-forming capacity was not impacted. We benchmarked DARIC33 potency relative to CD19 CAR T cells to estimate a T cell dose for clinical testing. In addition, we integrated in vitro and preclinical in vivo drug concentration thresholds for off-on state transitions, as well as murine and human rapamycin pharmacokinetics, to estimate a clinically applicable rapamycin dosing schedule. A phase I DARIC33 trial has been initiated (PLAT-08, NCT05105152), with initial evidence of rapamycin-regulated T cell activation and antitumor impact. Our findings provide evidence that the DARIC platform exhibits sensitive regulation and potency needed for clinical application to other important immunotherapy targets.
Keywords: Cancer immunotherapy; Hematology; Leukemias; T cells; Therapeutics.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

