Evaluating immunotherapeutic outcomes in triple-negative breast cancer with a cholesterol radiotracer in mice
- PMID: 38502228
- PMCID: PMC11141879
- DOI: 10.1172/jci.insight.175320
Evaluating immunotherapeutic outcomes in triple-negative breast cancer with a cholesterol radiotracer in mice
Abstract
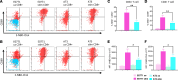
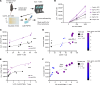
Evaluating the response to immune checkpoint inhibitors (ICIs) remains an unmet challenge in triple-negative breast cancer (TNBC). The requirement for cholesterol in the activation and function of T cells led us to hypothesize that quantifying cellular accumulation of this molecule could distinguish successful from ineffective checkpoint immunotherapy. To analyze accumulation of cholesterol by T cells in the immune microenvironment of breast cancer, we leveraged the PET radiotracer, eFNP-59. eFNP-59 is an analog of cholesterol that our group validated as an imaging biomarker for cholesterol uptake in preclinical models and initial human studies. In immunocompetent mouse models of TNBC, we found that elevated uptake of exogenous labeled cholesterol analogs functions as a marker for T cell activation. When comparing ICI-responsive and -nonresponsive tumors directly, uptake of fluorescent cholesterol and eFNP-59 increased in T cells from ICI-responsive tumors. We discovered that accumulation of cholesterol by T cells increased in ICI-responding tumors that received anti-PD-1 checkpoint immunotherapy. In patients with TNBC, tumors containing cycling T cells had features of cholesterol uptake and trafficking within those populations. These results suggest that uptake of exogenous cholesterol analogs by tumor-infiltrating T cells allows detection of T cell activation and has potential to assess the success of ICI therapy.
Keywords: Breast cancer; Diagnostic imaging; Immunology; Immunotherapy; Oncology.
Conflict of interest statement
Figures




References
-
- Cortes J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. doi: 10.1016/S0140-6736(20)32531-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

