STING activation reprograms the microenvironment to sensitize NF1-related malignant peripheral nerve sheath tumors for immunotherapy
- PMID: 38502231
- PMCID: PMC11093615
- DOI: 10.1172/JCI176748
STING activation reprograms the microenvironment to sensitize NF1-related malignant peripheral nerve sheath tumors for immunotherapy
Abstract
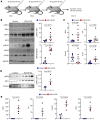
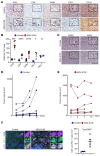
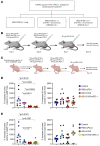
Neurofibromatosis type 1 (NF1) is caused by mutations in the NF1 gene that encodes neurofibromin, a RAS GTPase-activating protein. Inactivating NF1 mutations cause hyperactivation of RAS-mediated signaling, resulting in the development of multiple neoplasms, including malignant peripheral nerve sheath tumors (MPNSTs). MPNSTs are an aggressive tumor and the main cause of mortality in patients with NF1. MPNSTs are difficult to resect and refractory to chemo- and radiotherapy, and no molecular therapies currently exist. Immune checkpoint blockade (ICB) is an approach to treat inoperable, undruggable cancers like MPNST, but successful outcomes require an immune cell-rich tumor microenvironment. While MPNSTs are noninflamed "cold" tumors, here, we converted MPNSTs into T cell-inflamed "hot" tumors by activating stimulator of IFN genes (STING) signaling. Mouse genetic and human xenograft MPNST models treated with a STING agonist plus ICB exhibited growth delay via increased apoptotic cell death. This strategy offers a potential treatment regimen for MPNSTs.
Keywords: Cancer immunotherapy; Immunotherapy; Oncology; Therapeutics.
Conflict of interest statement
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

