Dysregulated fibrinolysis and plasmin activation promote the pathogenesis of osteoarthritis
- PMID: 38502232
- PMCID: PMC11141881
- DOI: 10.1172/jci.insight.173603
Dysregulated fibrinolysis and plasmin activation promote the pathogenesis of osteoarthritis
Abstract
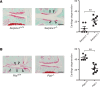
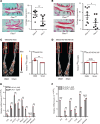
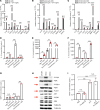
Joint injury is associated with risk for development of osteoarthritis (OA). Increasing evidence suggests that activation of fibrinolysis is involved in OA pathogenesis. However, the role of the fibrinolytic pathway is not well understood. Here, we showed that the fibrinolytic pathway, which includes plasminogen/plasmin, tissue plasminogen activator, urokinase plasminogen activator (uPA), and the uPA receptor (uPAR), was dysregulated in human OA joints. Pharmacological inhibition of plasmin attenuated OA progression after a destabilization of the medial meniscus in a mouse model whereas genetic deficiency of plasmin activator inhibitor, or injection of plasmin, exacerbated OA. We detected increased uptake of uPA/uPAR in mouse OA joints by microPET/CT imaging. In vitro studies identified that plasmin promotes OA development through multiple mechanisms, including the degradation of lubricin and cartilage proteoglycans and induction of inflammatory and degradative mediators. We showed that uPA and uPAR produced inflammatory and degradative mediators by activating the PI3K, 3'-phosphoinositide-dependent kinase-1, AKT, and ERK signaling cascades and activated matrix metalloproteinases to degrade proteoglycan. Together, we demonstrated that fibrinolysis contributes to the development of OA through multiple mechanisms and suggested that therapeutic targeting of the fibrinolysis pathway can prevent or slow development of OA.
Keywords: Inflammation; Osteoarthritis.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

