An Evidence-Based Update on Anticholinergic Use for Drug-Induced Movement Disorders
- PMID: 38502289
- PMCID: PMC10980662
- DOI: 10.1007/s40263-024-01078-z
An Evidence-Based Update on Anticholinergic Use for Drug-Induced Movement Disorders
Abstract
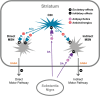
Drug-induced movement disorders (DIMDs) are associated with use of dopamine receptor blocking agents (DRBAs), including antipsychotics. The most common forms are drug-induced parkinsonism (DIP), dystonia, akathisia, and tardive dyskinesia (TD). Although rare, neuroleptic malignant syndrome (NMS) is a potentially life-threatening consequence of DRBA exposure. Recommendations for anticholinergic use in patients with DIMDs were developed on the basis of a roundtable discussion with healthcare professionals with extensive expertise in DIMD management, along with a comprehensive literature review. The roundtable agreed that "extrapyramidal symptoms" is a non-specific term that encompasses a range of abnormal movements. As such, it contributes to a misconception that all DIMDs can be treated in the same way, potentially leading to the misuse and overprescribing of anticholinergics. DIMDs are neurobiologically and clinically distinct, with different treatment paradigms and varying levels of evidence for anticholinergic use. Whereas evidence indicates anticholinergics can be effective for DIP and dystonia, they are not recommended for TD, akathisia, or NMS; nor are they supported for preventing DIMDs except in individuals at high risk for acute dystonia. Anticholinergics may induce serious peripheral adverse effects (e.g., urinary retention) and central effects (e.g., impaired cognition), all of which can be highly concerning especially in older adults. Appropriate use of anticholinergics therefore requires careful consideration of the evidence for efficacy (e.g., supportive for DIP but not TD) and the risks for serious adverse events. If used, anticholinergic medications should be prescribed at the lowest effective dose and for limited periods of time. When discontinued, they should be tapered gradually.
© 2024. The Author(s).
Conflict of interest statement
N.V.A. has received consulting honoraria from Neurocrine Biosciences and research support from the National Institutes of Health and the Parkinson’s Disease Foundation. S.N.C. has served as a consultant to Neurocrine Biosciences, Teva Pharmaceuticals, Osmotica Pharmaceuticals, and Dispersol Technologies. S.N.C. has received separate research grants from Neurocrine Biosciences, Osmotica Pharmaceuticals, and Eagle Pharmaceuticals. L.C. has received consulting fees from: AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, Biogen, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, Inmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Luye, Lyndra, MapLight, Marvin, Medavante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neumora, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sumitomo/Sunovion, Supernus, Teva, University of Arizona, Vanda, Wells Fargo, and one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; is a speaker for: AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies; owns stocks (small number of shares of common stock) in: Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer purchased > 10 years ago, stock options: Reviva; and receives royalties/publishing income from: Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022-date), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics) J.C. has been a consultant or advisor to Neurocrine Biosciences, Sunovion, Takeda, AstraZeneca, Otsuka, Janssen, Actavis (now AbbVie), Lundbeck, and Alkermes. R.S.M. has received research support from the Global Alliance for Chronic Diseases, Canadian Institutes of Health Research, National Natural Science Foundation of China-Mental Health Team, and the Milken Institute, and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine Biosciences, Neurawell, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris Pharma, Sanofi, Eisai, Intra-Cellular Therapies, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences. R.S.M. is the CEO of Braxia Scientific Corp. J.M.M. has served as a speaker or advisor for Alkermes, AbbVie, Axsome, BioXcel, Cerevel, Intra-Cellular Therapies, Karuna, Neurocrine Biosciences, Noven Pharmaceuticals, Otsuka America, Inc., Relmada, Sunovion, and Teva Pharmaceuticals. A.P. has received consultant or speaker fees from Neurocrine Biosciences, Avanir Pharmaceuticals, Acadia Pharmaceuticals, Otsuka, Allergan (now AbbVie), Teva Pharmaceuticals, AstraZeneca, and Takeda. J.M.S. has received consulting fees from Neurocrine Biosciences. K.F., R.M., and S.A.C. are full-time employees of Neurocrine Biosciences, Inc. L.L. is a former full-time employee of Neurocrine Biosciences.
Figures


References
-
- Stagnitti MN. Trends in antipsychotics purchases and expenses for the U.S. civilian noninstitutionalized population, 1997 and 2007. Statistical Brief #275. Agency for Healthcare Research and Quality, Rockville, MD. 2010.
-
- Ahrnsbra R, Stagnitti MN. Comparision of antidepressant and antipsychotic utilization and expenditures in the U.S. civilian noninstitutionalized population, 2013 and 2018. Statistical Brief #534. Agency for Healthcare Research and Quality, Rockville, MD. 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

