Establishment of a lethal mouse model of emerging tick-borne orthonairovirus infections
- PMID: 38502642
- PMCID: PMC10980201
- DOI: 10.1371/journal.ppat.1012101
Establishment of a lethal mouse model of emerging tick-borne orthonairovirus infections
Abstract
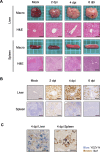
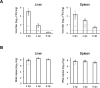
Emerging and reemerging tick-borne virus infections caused by orthonairoviruses (family Nairoviridae), which are genetically distinct from Crimean-Congo hemorrhagic fever virus, have been recently reported in East Asia. Here, we have established a mouse infection model using type-I/II interferon receptor-knockout mice (AG129 mice) both for a better understanding of the pathogenesis of these infections and validation of antiviral agents using Yezo virus (YEZV), a novel orthonairovirus causing febrile illnesses associated with tick bites in Japan and China. YEZV-inoculated AG129 mice developed hepatitis with body weight loss and died by 6 days post infection. Blood biochemistry tests showed elevated liver enzyme levels, similar to YEZV-infected human patients. AG129 mice treated with favipiravir survived lethal YEZV infection, demonstrating the anti-YEZV effect of this drug. The present mouse model will help us better understand the pathogenicity of the emerging tick-borne orthonairoviruses and the development of specific antiviral agents for their treatment.
Copyright: © 2024 Ariizumi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Burt FJ, Spencer DC, Leman PA, Patterson B, Swanepoel R. Investigation of tick-borne viruses as pathogens of humans in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol Infect. 1996;116: 353–361. doi: 10.1017/s0950268800052687 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

