Exercise-induced BDNF promotes PPARδ-dependent reprogramming of lipid metabolism in skeletal muscle during exercise recovery
- PMID: 38502732
- PMCID: PMC11022078
- DOI: 10.1126/scisignal.adh2783
Exercise-induced BDNF promotes PPARδ-dependent reprogramming of lipid metabolism in skeletal muscle during exercise recovery
Abstract
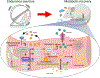
Post-exercise recovery is essential to resolve metabolic perturbations and promote long-term cellular remodeling in response to exercise. Here, we report that muscle-generated brain-derived neurotrophic factor (BDNF) elicits post-exercise recovery and metabolic reprogramming in skeletal muscle. BDNF increased the post-exercise expression of the gene encoding PPARδ (peroxisome proliferator-activated receptor δ), a transcription factor that is a master regulator of lipid metabolism. After exercise, mice with muscle-specific Bdnf knockout (MBKO) exhibited impairments in PPARδ-regulated metabolic gene expression, decreased intramuscular lipid content, reduced β-oxidation, and dysregulated mitochondrial dynamics. Moreover, MBKO mice required a longer period to recover from a bout of exercise and did not show increases in exercise-induced endurance capacity. Feeding naïve mice with the bioavailable BDNF mimetic 7,8-dihydroxyflavone resulted in effects that mimicked exercise-induced adaptations, including improved exercise capacity. Together, our findings reveal that BDNF is an essential myokine for exercise-induced metabolic recovery and remodeling in skeletal muscle.
Conflict of interest statement
COMPETING INTEREST
The authors declare that they have no competing interests.
Figures






References
-
- van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH, Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553, 611–625 (2003); published online EpubDec 1 (10.1113/jphysiol.2003.052431). - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

