Morusin Alleviates Aortic Valve Calcification by Inhibiting Valve Interstitial Cell Senescence Through Ccnd1/Trim25/Nrf2 Axis
- PMID: 38502885
- PMCID: PMC11132047
- DOI: 10.1002/advs.202307319
Morusin Alleviates Aortic Valve Calcification by Inhibiting Valve Interstitial Cell Senescence Through Ccnd1/Trim25/Nrf2 Axis
Abstract
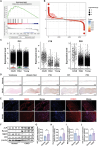
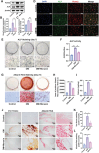
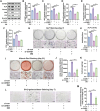
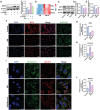
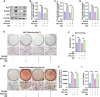
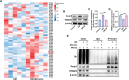
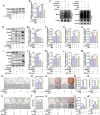
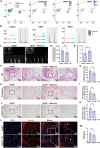
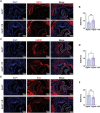
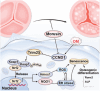
The senescence of aortic valve interstitial cells (VICs) plays a critical role in the progression of calcific aortic valve disease (CAVD). However, the precise mechanisms underlying the senescence of VICs remain unclear, demanding the identification of a novel target to mitigate this process. Previous studies have highlighted the anti-aging potential of morusin. Thus, this study aimed to explore the therapeutic potential of morusin in CAVD. Cellular experiments reveal that morusin effectively suppresses cellular senescence and cause a shift toward osteogenic differentiation of VICs in vitro. Mechanistically, morusin activate the Nrf2-mediated antiaging signaling pathway by downregulating CCND1 expression and aiding Keap1 degradation through Trim 25. This activation lead to the upregulated expression of antioxidant genes, thus reducing reactive oxygen species production and thereby preventing VIC osteogenic differentiation. In vivo experiments in ApoE-/- mice on a high-fat Western diet demonstrate the positive effect of morusin in mitigating aortic valve calcification. These findings emphasize the antiaging properties of morusin and its potential as a therapeutic agent for CAVD.
Keywords: antiaging; calcific aortic valve disease; molecular target; natural product.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yadgir S., Johnson C. O., Aboyans V., Adebayo O. M., Adedoyin R. A., Afarideh M., Alahdab F., Alashi A., Alipour V., Arabloo J., Azari S., Barthelemy C. M., Benziger C. P., Berman A. E., Bijani A., Carrero J. J., Carvalho F., Daryani A., Duraes A. R., Esteghamati A., Farid T. A., Farzadfar F., Fernandes E., Filip I., Gad M. M., Hamidi S., Hay S. I., Ilesanmi O. S., Naghibi Irvani S. S., Jurisson M., Circulation 2020, 141, 1670 . - PubMed
-
- Xu K., Xie S., Huang Y., Zhou T., Liu M., Zhu P., Wang C., Shi J., Li F., Sellke F. W., Dong N., Arterioscler Thromb Vasc Biol 2020, 40, 2910. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
