Patient Preferences for Treatment Attributes in Inflammatory Bowel Disease: Results From a Large Survey Across Seven European Countries Using a Discrete Choice Experiment
- PMID: 38503480
- PMCID: PMC11630295
- DOI: 10.1093/ibd/izae015
Patient Preferences for Treatment Attributes in Inflammatory Bowel Disease: Results From a Large Survey Across Seven European Countries Using a Discrete Choice Experiment
Abstract
Background: Inflammatory bowel disease requires long-term treatment; therefore, understanding patient preferences is important in aiding informed treatment decision making. This study explored patients' preferences for treatment attributes of available inflammatory bowel disease therapies.
Methods: Adult patients from 7 European countries who self-reported previous/current treatment for Crohn's disease (CD) or ulcerative colitis (UC) participated in an online survey via the Carenity platform. In a discrete choice experiment, the relative importance of treatment attributes for CD and UC was estimated using conditional logit models. Latent class analysis was conducted to estimate heterogeneous treatment preferences based on patient profiles. Patients' perspectives and preferences regarding their quality of life were assessed.
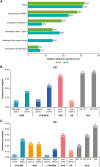
Results: Across 686 completed survey responses (CD, n = 360; UC, n = 326), the mean patient age was 48 and 50 years, respectively. Patients with CD ranked route of administration as the most important attribute (attribute importance: 32%), preferring subcutaneous over intravenous treatment (P < .001). Patients with UC ranked route of administration and frequency of serious adverse events as the most important attributes (attribute importance: 31% and 23%, respectively), preferring oral (P < .001) and subcutaneous (P < .001) over intravenous treatment and treatment that minimized the risk of serious adverse events (P < .001) or mild adverse events (P < .01). Latent class analyses confirmed the impact of patients' sociodemographic profile on their preferences. All patients prioritized general well-being, energy level, and daily activities as the most important aspects for improvement through treatment.
Conclusions: Patient preferences for treatment attributes varied among patients with CD or UC, highlighting the importance of personalized care and shared decision making to maximize treatment benefits.
Keywords: discrete choice experiment; inflammatory bowel disease; patient preference.
Plain language summary
This study explored patients’ preferences for treatment attributes in Crohn’s disease or ulcerative colitis, such as subcutaneous/intravenous drug administration and adverse effects. Patients’ preferences highlighted the importance of personalized care and shared decision making to maximize treatment benefits.
© 2024 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
G.F. has received consultancy fees from MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, Alfasigma, Samsung, Amgen, Roche, Galapagos, and Ferring. N.B.-E. is an employee of Takeda and holds Takeda stock options. F.B. is a former employee of Takeda. P.V. is a former employee of Carenity. E.H. has received consultancy fees from Galapagos and Takeda and speakers fees from Celltrion.
Figures




Similar articles
-
Patient Preferences for Treatment Attributes in Inflammatory Bowel Disease: A Discrete Choice Experiment Among Patients in Five Non-Western Countries.Adv Ther. 2025 Aug;42(8):3922-3944. doi: 10.1007/s12325-025-03249-w. Epub 2025 Jun 17. Adv Ther. 2025. PMID: 40526254 Free PMC article.
-
Inflammatory Bowel Disease Patients' Treatment Preferences Using a Discrete Choice Experiment Technique: The InPuT Study.Adv Ther. 2022 Jun;39(6):2889-2905. doi: 10.1007/s12325-022-02143-z. Epub 2022 Apr 22. Adv Ther. 2022. PMID: 35451740 Free PMC article.
-
Optimizing Selection of Biologics in Inflammatory Bowel Disease: Development of an Online Patient Decision Aid Using Conjoint Analysis.Am J Gastroenterol. 2018 Jan;113(1):58-71. doi: 10.1038/ajg.2017.470. Epub 2017 Dec 5. Am J Gastroenterol. 2018. PMID: 29206816
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2023 Apr 25;4(4):CD012774. doi: 10.1002/14651858.CD012774.pub3. Cochrane Database Syst Rev. 2023. PMID: 37094824 Free PMC article. Review.
-
Assessing patient preferences for treatment options and process of care in inflammatory bowel disease: a critical review of quantitative data.Patient. 2013;6(4):241-55. doi: 10.1007/s40271-013-0031-2. Patient. 2013. PMID: 24127239 Free PMC article. Review.
Cited by
-
Discrete choice experiment on the preferences for continuing medical education training programs among primary health care physicians in China.BMC Med Educ. 2025 Feb 26;25(1):315. doi: 10.1186/s12909-025-06828-1. BMC Med Educ. 2025. PMID: 40011922 Free PMC article.
-
Patient and Healthcare Professional Satisfaction, Acceptability, and Preference Experiences With Mirikizumab Administration for Ulcerative Colitis: An International Survey.Crohns Colitis 360. 2024 Oct 4;6(4):otae054. doi: 10.1093/crocol/otae054. eCollection 2024 Oct. Crohns Colitis 360. 2024. PMID: 39473630 Free PMC article.
-
Patient Preferences for Treatment Attributes in Inflammatory Bowel Disease: A Discrete Choice Experiment Among Patients in Five Non-Western Countries.Adv Ther. 2025 Aug;42(8):3922-3944. doi: 10.1007/s12325-025-03249-w. Epub 2025 Jun 17. Adv Ther. 2025. PMID: 40526254 Free PMC article.
-
Patients' and gastroenterologists' preferences regarding outcomes and medication attributes in ulcerative colitis.Ann Gastroenterol. 2025 Mar-Apr;38(2):174-181. doi: 10.20524/aog.2025.0944. Epub 2025 Feb 25. Ann Gastroenterol. 2025. PMID: 40124436 Free PMC article.
References
-
- Burisch J, Jess T, Martinato M, Lakatos PL; ECCO -EpiCom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7(4):322-337. - PubMed
-
- Coward S, Clement F, Benchimol EI, et al.Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156(5):1345-1353.e4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

