RAPP-containing arrest peptides induce translational stalling by short circuiting the ribosomal peptidyltransferase activity
- PMID: 38503735
- PMCID: PMC10951233
- DOI: 10.1038/s41467-024-46761-3
RAPP-containing arrest peptides induce translational stalling by short circuiting the ribosomal peptidyltransferase activity
Erratum in
-
Publisher Correction: RAPP-containing arrest peptides induce translational stalling by short circuiting the ribosomal peptidyltransferase activity.Nat Commun. 2024 Apr 15;15(1):3242. doi: 10.1038/s41467-024-47508-w. Nat Commun. 2024. PMID: 38622122 Free PMC article. No abstract available.
Abstract
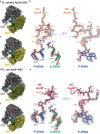
Arrest peptides containing RAPP (ArgAlaProPro) motifs have been discovered in both Gram-positive and Gram-negative bacteria, where they are thought to regulate expression of important protein localization machinery components. Here we determine cryo-EM structures of ribosomes stalled on RAPP arrest motifs in both Bacillus subtilis and Escherichia coli. Together with molecular dynamics simulations, our structures reveal that the RAPP motifs allow full accommodation of the A-site tRNA, but prevent the subsequent peptide bond from forming. Our data support a model where the RAP in the P-site interacts and stabilizes a single hydrogen atom on the Pro-tRNA in the A-site, thereby preventing an optimal geometry for the nucleophilic attack required for peptide bond formation to occur. This mechanism to short circuit the ribosomal peptidyltransferase activity is likely to operate for the majority of other RAPP-like arrest peptides found across diverse bacterial phylogenies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

