The bone ecosystem facilitates multiple myeloma relapse and the evolution of heterogeneous drug resistant disease
- PMID: 38503736
- PMCID: PMC10951361
- DOI: 10.1038/s41467-024-46594-0
The bone ecosystem facilitates multiple myeloma relapse and the evolution of heterogeneous drug resistant disease
Abstract
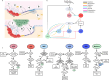
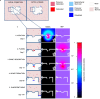
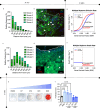
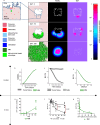
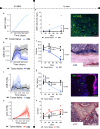
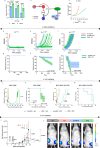
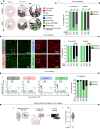
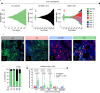
Multiple myeloma (MM) is an osteolytic malignancy that is incurable due to the emergence of treatment resistant disease. Defining how, when and where myeloma cell intrinsic and extrinsic bone microenvironmental mechanisms cause relapse is challenging with current biological approaches. Here, we report a biology-driven spatiotemporal hybrid agent-based model of the MM-bone microenvironment. Results indicate MM intrinsic mechanisms drive the evolution of treatment resistant disease but that the protective effects of bone microenvironment mediated drug resistance (EMDR) significantly enhances the probability and heterogeneity of resistant clones arising under treatment. Further, the model predicts that targeting of EMDR deepens therapy response by eliminating sensitive clones proximal to stroma and bone, a finding supported by in vivo studies. Altogether, our model allows for the study of MM clonal evolution over time in the bone microenvironment and will be beneficial for optimizing treatment efficacy so as to significantly delay disease relapse.
© 2024. The Author(s).
Conflict of interest statement
K.H.S. reports honoraria from Bristol Myers Squibb, Janssen, Adaptive Biotechnology, Sanofi, GlaxoSmithKline, Takeda, Amgen, and Sebia as well as research funding to the institution from AbbVie and Karyopharm. All the other authors have no competing interests.
Figures
References
-
- Howlader N. N. A., et al. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. (2019).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

