Vagus nerve stimulation as a promising neuroprotection for ischemic stroke via α7nAchR-dependent inactivation of microglial NLRP3 inflammasome
- PMID: 38504011
- PMCID: PMC11192746
- DOI: 10.1038/s41401-024-01245-4
Vagus nerve stimulation as a promising neuroprotection for ischemic stroke via α7nAchR-dependent inactivation of microglial NLRP3 inflammasome
Abstract
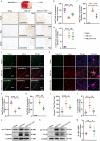
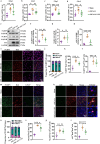
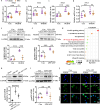
Ischemic stroke is a major cause of disability and death worldwide, and its management requires urgent attention. Previous studies have shown that vagus nerve stimulation (VNS) exerts neuroprotection in ischemic stroke by inhibiting neuroinflammation and apoptosis. In this study, we evaluated the timing for VNS intervention in ischemic stroke, and the underlying mechanisms of VNS-induced neuroprotection. Mice were subjected to transient middle cerebral artery occlusion (tMCAO) for 60 min. The left vagus nerve at cervical level was exposed and attached to an electrode connected to a low-frequency electrical stimulator. Vagus nerve stimulation (VNS) was given for 60 min before, during and after tMCAO (Pre-VNS, Dur-VNS, Post-VNS). Neurological function was assessed 24 h after reperfusion. We found that all the three VNS significantly protected against the tMCAO-induced injury evidenced by improved neurological function and reduced infarct volume. Moreover, the Pre-VNS was the most effective against the ischemic injury. We found that tMCAO activated microglia in the ischemic core and penumbra regions of the brain, followed by the NLRP3 inflammasome activation-induced neuroinflammation, which finally triggered neuronal death. VNS treatment preserved α7nAChR expression in the penumbra regions, inhibited NLRP3 inflammasome activation and ensuing neuroinflammation, rescuing cerebral neurons. The role of α7nAChR in microglial NLRP3 inflammasome activation in ischemic stroke was further validated using genetic manipulations, including Chrna7 knockout mice and microglial Chrna7 overexpression mice, as well as pharmacological interventions using the α7nAChR inhibitor methyllycaconitine and agonist PNU-282987. Collectively, this study demonstrates the potential of VNS as a safe and effective strategy to treat ischemic stroke, and presents a new approach targeting microglial NLRP3 inflammasome, which might be therapeutic for other inflammation-related diseases.
Keywords: NLRP3; ischemic stroke; microglia; neuroinflammation; vagus nerve stimulation; α7 nicotinic acetylcholine receptor.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

