A visual-language foundation model for computational pathology
- PMID: 38504017
- PMCID: PMC11384335
- DOI: 10.1038/s41591-024-02856-4
A visual-language foundation model for computational pathology
Abstract
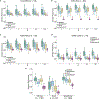
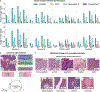
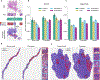
The accelerated adoption of digital pathology and advances in deep learning have enabled the development of robust models for various pathology tasks across a diverse array of diseases and patient cohorts. However, model training is often difficult due to label scarcity in the medical domain, and a model's usage is limited by the specific task and disease for which it is trained. Additionally, most models in histopathology leverage only image data, a stark contrast to how humans teach each other and reason about histopathologic entities. We introduce CONtrastive learning from Captions for Histopathology (CONCH), a visual-language foundation model developed using diverse sources of histopathology images, biomedical text and, notably, over 1.17 million image-caption pairs through task-agnostic pretraining. Evaluated on a suite of 14 diverse benchmarks, CONCH can be transferred to a wide range of downstream tasks involving histopathology images and/or text, achieving state-of-the-art performance on histology image classification, segmentation, captioning, and text-to-image and image-to-text retrieval. CONCH represents a substantial leap over concurrent visual-language pretrained systems for histopathology, with the potential to directly facilitate a wide array of machine learning-based workflows requiring minimal or no further supervised fine-tuning.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
M.Y.L., B.C., R.J.C. and F.M. are inventors on a provisional US patent (application number 63/610,645) filed corresponding to the methodological aspects of this work.
Figures





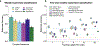






References
-
- Song AH et al. Artificial intelligence for digital and computational pathology. Nat. Rev. Bioeng. 1, 930–949 (2023).
-
- Shmatko A, Ghaffari Laleh N, Gerstung M & Kather JN Artificial intelligence in histopathology: enhancing cancer research and clinical oncology. Nat. Cancer 3, 1026–1038 (2022). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

