A topographical atlas of α-synuclein dosage and cell type-specific expression in adult mouse brain and peripheral organs
- PMID: 38504090
- PMCID: PMC10951202
- DOI: 10.1038/s41531-024-00672-8
A topographical atlas of α-synuclein dosage and cell type-specific expression in adult mouse brain and peripheral organs
Abstract
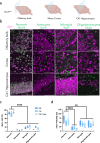
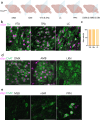
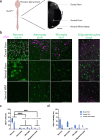
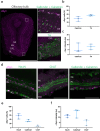
Parkinson's disease (PD) is the second most common neurodegenerative disease worldwide and presents pathologically with Lewy pathology and dopaminergic neurodegeneration. Lewy pathology contains aggregated α-synuclein (αSyn), a protein encoded by the SNCA gene which is also mutated or duplicated in a subset of familial PD cases. Due to its predominant presynaptic localization, immunostaining for the protein results in a diffuse reactivity pattern, providing little insight into the types of cells expressing αSyn. As a result, insight into αSyn expression-driven cellular vulnerability has been difficult to ascertain. Using a combination of knock-in mice that target αSyn to the nucleus (SncaNLS) and in situ hybridization of Snca in wild-type mice, we systematically mapped the topography and cell types expressing αSyn in the mouse brain, spinal cord, retina, and gut. We find a high degree of correlation between αSyn protein and RNA levels and further identify cell types with low and high αSyn content. We also find high αSyn expression in neurons, particularly those involved in PD, and to a lower extent in non-neuronal cell types, notably those of oligodendrocyte lineage, which are relevant to multiple system atrophy pathogenesis. Surprisingly, we also found that αSyn is relatively absent from select neuron types, e.g., ChAT-positive motor neurons, whereas enteric neurons universally express some degree of αSyn. Together, this integrated atlas provides insight into the cellular topography of αSyn, and provides a quantitative map to test hypotheses about the role of αSyn in network vulnerability, and thus serves investigations into PD pathogenesis and other α-synucleinopathies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

