Clinical effectiveness of a multitarget urine DNA test for urothelial carcinoma detection: a double-blinded, multicenter, prospective trial
- PMID: 38504268
- PMCID: PMC10949661
- DOI: 10.1186/s12943-024-01974-4
Clinical effectiveness of a multitarget urine DNA test for urothelial carcinoma detection: a double-blinded, multicenter, prospective trial
Abstract
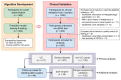
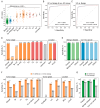
Urine-based testing is promising for noninvasive diagnosis of urothelial carcinoma (UC) but has suboptimal sensitivity for early-stage tumors. Herein, we developed a multitarget urine tumor DNA test, UI-Seek, for UC detection and evaluated its clinical feasibility. The prediction model was developed in a retrospective cohort (n = 382), integrating assays for FGFR3 and TERT mutations and aberrant ONECUT2 and VIM methylation to generate a UC-score. The test performance was validated in a double-blinded, multicenter, prospective trial (n = 947; ChiCTR2300076543) and demonstrated a sensitivity of 91.37% and a specificity of 95.09%. The sensitivity reached 75.81% for low-grade Ta tumors and exceeded 93% in high-grade Ta and higher stages (T1 to T4). Simultaneous identification of both bladder and upper urinary tract tumors was enabled with sensitivities exceeding 90%. No significant confounding effects were observed regarding benign urological diseases or non-UC malignancies. The test showed improved sensitivities over urine cytology, the NMP22 test, and UroVysion FISH alongside comparable specificities. The single-target accuracy was greater than 98% as confirmed by Sanger sequencing. Post-surgery UC-score decreased in 97.7% of subjects. Overall, UI-Seek demonstrated robust performance and considerable potential for the early detection of UC.
Keywords: Cancer biomarkers; Early detection; Liquid biopsy; Molecular diagnosis; Urine tumor DNA; Urothelial carcinoma.
© 2024. The Author(s).
Conflict of interest statement
X.L., H.W., T.Y., R.T., J.G., L.C., H.C., F.L., and S.C. are current employees of Acornmed Biotechnology Co., Ltd. The remaining authors declare that they have no competing interests.
Figures
References
-
- Statistics adapted from the American Cancer Society’s (ACS) publication, Cancer Facts & Figures 2023; the ACS website; and the International Agency for Research on Cancer website. (All sources accessed February 2023.). https://www.cancer.net/cancer-types/bladder-cancer/statistics (2023). Accessed 10 Aug 2023.
-
- NCCN Clinical Practice Guidelines in Oncology-Bladder Cancer. (Version 2.2023). http://www.nccn.org (2023). Accessed 10 Aug 2023.
Publication types
MeSH terms
Substances
Grants and funding
- TJWJ2022XK014/Tianjin Municipal Health Industry Key Project
- 2022ZD069/Scientific Research Project of Tianjin Municipal Education Commission
- MYSRC202310/Tianjin Institute of Urology Talent Funding Program
- TJYXZDXK-023A/Tianjin Key Medical Discipline (Specialty) Construction Project
- Y-Young2023-0087/Zhaoyang Cancer Research Fund of The Chinese Society of Clinical Oncology
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

