Unveiling CXCR2 as a promising therapeutic target in renal cell carcinoma: exploring the immunotherapeutic paradigm shift through its inhibition by RCT001
- PMID: 38504270
- PMCID: PMC10949812
- DOI: 10.1186/s13046-024-02984-2
Unveiling CXCR2 as a promising therapeutic target in renal cell carcinoma: exploring the immunotherapeutic paradigm shift through its inhibition by RCT001
Abstract
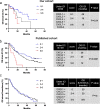
Background: In clear cell renal cell carcinoma (ccRCC), first-line treatment combines nivolumab (anti-PD-1) and ipilimumab (anti-CTLA4), yielding long-term remissions but with only a 40% success rate. Our study explored the potential of enhancing ccRCC treatment by concurrently using CXCR2 inhibitors alongside immunotherapies.
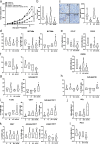
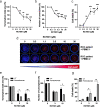
Methods: We analyzed ELR + CXCL levels and their correlation with patient survival during immunotherapy. RCT001, a unique CXCR2 inhibitor, was examined for its mechanism of action, particularly its effects on human primary macrophages. We tested the synergistic impact of RCT001 in combination with immunotherapies in both mouse models of ccRCC and human ccRCC in the presence of human PBMC.
Resuts: Elevated ELR + CXCL cytokine levels were found to correlate with reduced overall survival during immunotherapy. RCT001, our optimized compound, acted as an inverse agonist, effectively inhibiting angiogenesis and reducing viability of primary ccRCC cells. It redirected M2-like macrophages without affecting M1-like macrophage polarization directed against the tumor. In mouse models, RCT001 enhanced the efficacy of anti-CTLA4 + anti-PD1 by inhibiting tumor-associated M2 macrophages and tumor-associated neutrophils. It also impacted the activation of CD4 T lymphocytes, reducing immune-tolerant lymphocytes while increasing activated natural killer and dendritic cells. Similar effectiveness was observed in human RCC tumors when RCT001 was combined with anti-PD-1 treatment.
Conclusions: RCT001, by inhibiting CXCR2 through its unique mechanism, effectively suppresses ccRCC cell proliferation, angiogenesis, and M2 macrophage polarization. This optimization potentiates the efficacy of immunotherapy and holds promise for significantly improving the survival prospects of metastatic ccRCC patients.
Keywords: CXCR2 inhibitors; Immunotherapies resistance; Ipilimumab; M2 tumor associated macrophages; Nivolumab; Renal Cell Carcinoma.
© 2024. The Author(s).
Conflict of interest statement
We disclose that RB, CR, GP, MD are the co-founders of the start-up Roca Therapeutics who develops RCT001.
Figures



References
-
- Padala SA, Kallam A. Clear Cell Renal Carcinoma. StatPearls. Treasure Island (FL)2023. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

