Exploring the role of genetic variations in NAFLD: implications for disease pathogenesis and precision medicine approaches
- PMID: 38504356
- PMCID: PMC10953212
- DOI: 10.1186/s40001-024-01708-8
Exploring the role of genetic variations in NAFLD: implications for disease pathogenesis and precision medicine approaches
Abstract
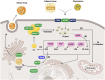
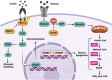
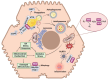
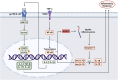
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver diseases, affecting more than one-quarter of people worldwide. Hepatic steatosis can progress to more severe forms of NAFLD, including NASH and cirrhosis. It also may develop secondary diseases such as diabetes and cardiovascular disease. Genetic and environmental factors regulate NAFLD incidence and progression, making it a complex disease. The contribution of various environmental risk factors, such as type 2 diabetes, obesity, hyperlipidemia, diet, and sedentary lifestyle, to the exacerbation of liver injury is highly understood. Nevertheless, the underlying mechanisms of genetic variations in the NAFLD occurrence or its deterioration still need to be clarified. Hence, understanding the genetic susceptibility to NAFLD is essential for controlling the course of the disease. The current review discusses genetics' role in the pathological pathways of NAFLD, including lipid and glucose metabolism, insulin resistance, cellular stresses, and immune responses. Additionally, it explains the role of the genetic components in the induction and progression of NAFLD in lean individuals. Finally, it highlights the utility of genetic knowledge in precision medicine for the early diagnosis and treatment of NAFLD patients.
Keywords: Gene variants; Lean NAFLD; NAFLD; NASH; Polymorphism; Precision medicine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis.Aliment Pharmacol Ther. 2020 Jun;51(12):1305-1320. doi: 10.1111/apt.15738. Epub 2020 May 7. Aliment Pharmacol Ther. 2020. PMID: 32383295 Free PMC article. Review.
-
Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age?World J Gastroenterol. 2014 Jul 21;20(27):9072-89. doi: 10.3748/wjg.v20.i27.9072. World J Gastroenterol. 2014. PMID: 25083080 Free PMC article. Review.
-
Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies.Lancet Diabetes Endocrinol. 2019 Apr;7(4):313-324. doi: 10.1016/S2213-8587(18)30154-2. Epub 2018 Aug 30. Lancet Diabetes Endocrinol. 2019. PMID: 30174213 Review.
-
Non-Alcoholic Fatty Liver Disease Treatment in Patients with Type 2 Diabetes Mellitus; New Kids on the Block.Curr Vasc Pharmacol. 2020;18(2):172-181. doi: 10.2174/1570161117666190405164313. Curr Vasc Pharmacol. 2020. PMID: 30961499 Review.
-
Genetics of Nonalcoholic Fatty Liver Disease: From Pathogenesis to Therapeutics.Semin Liver Dis. 2019 May;39(2):124-140. doi: 10.1055/s-0039-1679920. Epub 2019 Mar 25. Semin Liver Dis. 2019. PMID: 30912099 Review.
Cited by
-
Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases.Front Cell Dev Biol. 2024 Jul 16;12:1433857. doi: 10.3389/fcell.2024.1433857. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39086662 Free PMC article. Review.
-
From adiposity to steatosis: metabolic dysfunction-associated steatotic liver disease, a hepatic expression of metabolic syndrome - current insights and future directions.Clin Diabetes Endocrinol. 2024 Dec 2;10(1):39. doi: 10.1186/s40842-024-00187-4. Clin Diabetes Endocrinol. 2024. PMID: 39617908 Free PMC article. Review.
-
Comparison of wild-type and high-risk PNPLA3 variants in a human biomimetic liver microphysiology system for metabolic dysfunction-associated steatotic liver disease precision therapy.Front Cell Dev Biol. 2024 Sep 11;12:1423936. doi: 10.3389/fcell.2024.1423936. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39324073 Free PMC article.
-
Genotypic variation in CYP2E1, GCKR, and PNPLA3 among nonalcoholic steatohepatitis patients of Turkish origin.Mol Biol Rep. 2024 Jul 23;51(1):845. doi: 10.1007/s11033-024-09787-w. Mol Biol Rep. 2024. PMID: 39042259
-
Genetic variants associated with metabolic dysfunction-associated fatty liver diseases in a Korean population.Eur J Med Res. 2025 Apr 22;30(1):318. doi: 10.1186/s40001-025-02576-6. Eur J Med Res. 2025. PMID: 40264238 Free PMC article.
References
-
- Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. 2022;20(2):283–92.e10. doi: 10.1016/j.cgh.2021.05.002. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

