Effectiveness and safety of Jiawei Xiaoyao pill in the treatment of premenstrual syndrome (liver depression, spleen deficiency, and blood-heat syndrome): a multi-center, randomized, placebo-controlled trial
- PMID: 38504543
- PMCID: PMC10927397
- DOI: 10.19852/j.cnki.jtcm.20231110.003
Effectiveness and safety of Jiawei Xiaoyao pill in the treatment of premenstrual syndrome (liver depression, spleen deficiency, and blood-heat syndrome): a multi-center, randomized, placebo-controlled trial
Abstract
Objective: To investigate the effectiveness and safety of Jiawei Xiaoyao pill (,JXP) in the treatment of symptoms associated with premenstrual syndrome (PMS).
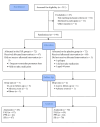
Methods: A total of 144 regularly menstruating women with PMS were recruited at 8 sites in China from August 2017 to December 2018, and randomized to receive either a JXP or a matching placebo (12 g/d, 6 g twice a day) for 3 menstrual cycles. The primary indicator was the reduced Daily Record of Severity of Problems (DRSP) scores in the luteal phase after 3 months of treatment. The safety outcomes included clinical adverse events (AEs), adverse reactions (ARs), changes in vital signs, and laboratory tests.
Results: JXP surpassed the placebo in reducing DRSP scores (psychological/somatic dysfunction) in the luteal phase over 3 menstrual cycles of treatment (PFAS = 0.002, PPPS = 0.001). Additionally, there were no significant differences in the incidence of AEs, severe AEs, withdrawal due to AEs and ARs between the two groups (all P > 0.05), and no clinically significant adverse medical events related to the test drug observed.
Conclusions: JXP was superior to the placebo in relieving the symptoms associated with PMS, which signified that JXP may be effective, safe, and well-tolerated as an alternative therapy.
Keywords: Jiawei Xiaoyao pill; double-blind method; premenstrual syndrome; randomized controlled trial.
Figures
References
-
- Korelo RIG, Moreira NB, Miguel BAC, et al. . Effects of auriculotherapy on treatment of women with premenstrual syndrome symptoms: a randomized, placebo-controlled clinical trial. Complement Ther Med 2022; 66: 102816. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical