Histaminergic System and Vestibular Function in Normal and Pathological Conditions
- PMID: 38504566
- PMCID: PMC11284731
- DOI: 10.2174/1570159X22666240319123151
Histaminergic System and Vestibular Function in Normal and Pathological Conditions
Abstract
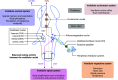
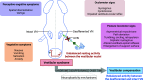
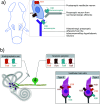
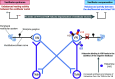
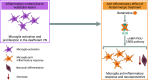
Most neurotransmitter systems are represented in the central and peripheral vestibular system and are thereby involved both in normal vestibular signal processing and the pathophysiology of vestibular disorders. However, there is a special relationship between the vestibular system and the histaminergic system. The purpose of this review is to document how the histaminergic system interferes with normal and pathological vestibular function. In particular, we will discuss neurobiological mechanisms such as neuroinflammation that involve histamine to modulate and allow restoration of balance function in the situation of a vestibular insult. These adaptive mechanisms represent targets of histaminergic pharmacological compounds capable of restoring vestibular function in pathological situations. The clinical use of drugs targeting the histaminergic system in various vestibular disorders is critically discussed.
Keywords: Vestibular system; histaminergic drugs; histaminergic system; neuroinflammation; vertigo.; vestibular disorders.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures



References
-
- Azzena G.B., Mameli O., Tolu E. Distribution of visual input to the vestibular nuclei. Arch. Ital. Biol. 1980;118(2):196–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
