Lessons for future clinical trials in adults with Becker muscular dystrophy: Disease progression detected by muscle magnetic resonance imaging, clinical and patient-reported outcome measures
- PMID: 38504654
- PMCID: PMC11235693
- DOI: 10.1111/ene.16282
Lessons for future clinical trials in adults with Becker muscular dystrophy: Disease progression detected by muscle magnetic resonance imaging, clinical and patient-reported outcome measures
Abstract
Background and purpose: Because Becker muscular dystrophy (BMD) is a heterogeneous disease and only few studies have evaluated adult patients, it is currently still unclear which outcome measures should be used in future clinical trials.
Methods: Muscle magnetic resonance imaging, patient-reported outcome measures and a wide range of clinical outcome measures, including motor function, muscle strength and timed-function tests, were evaluated in 21 adults with BMD at baseline and at 9 and 18 months of follow-up.
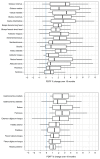
Results: Proton density fat fraction increased significantly in 10/17 thigh muscles after 9 months, and in all thigh and lower leg muscles after 18 months. The 32-item Motor Function Measurement (MFM-32) scale (-1.3%, p = 0.017), North Star Ambulatory Assessment (-1.3 points, p = 0.010) and patient-reported activity limitations scale (-0.3 logits, p = 0.018) deteriorated significantly after 9 months. The 6-min walk distance (-28.7 m, p = 0.042), 10-m walking test (-0.1 m/s, p = 0.032), time to climb four stairs test (-0.03 m/s, p = 0.028) and Biodex peak torque measurements of quadriceps (-4.6 N m, p = 0.014) and hamstrings (-5.0 N m, p = 0.019) additionally deteriorated significantly after 18 months. At this timepoint, domain 1 of the MFM-32 was the only clinical outcome measure with a large sensitivity to change (standardized response mean 1.15).
Discussion: It is concluded that proton density fat fraction imaging of entire thigh muscles is a sensitive outcome measure to track progressive muscle fat replacement in patients with BMD, already after 9 months of follow-up. Finally, significant changes are reported in a wide range of clinical and patient-reported outcome measures, of which the MFM-32 appeared to be the most sensitive to change in adults with BMD.
Keywords: BMD; Dixon; magnetic resonance imaging; quantitative MRI; trial readiness.
© 2024 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
KGC is Chairholder of the Emil von Behring Chair for Neuromuscular and Neurodegenerative Disorders by CSL Behring. Several authors are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO‐NMD) and the European Reference Network for Rare Neurological Diseases (ERN‐RND). ND has received compensation for lectures and/or advisory boards from Alnylam, Sanofi, Jansen and ArgenX. The authors report no disclosures relevant to the paper.
Figures


References
-
- Waldrop MA, Flanigan KM. Update in Duchenne and Becker muscular dystrophy. Curr Opin Neurol. 2019;32:722‐727. - PubMed
-
- Bushby KMD, Thambyayah M, Gardner‐Medwin D. Prevalence and incidence of Becker muscular dystrophy. Lancet. 1991;337:1022‐1024. - PubMed
-
- Fortunato F, Farnè M, Ferlini A. The DMD gene and therapeutic approaches to restore dystrophin. Neuromuscul Disord. 2021;31:1013‐1020. - PubMed
-
- Maggi L, Moscatelli M, Frangiamore R, et al. Quantitative muscle MRI protocol as possible biomarker in Becker muscular dystrophy. Clin Neuroradiol. 2021;31:257‐266. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

