NHC Catalysis for Umpolung Pyridinium Alkylation via Deoxy-Breslow Intermediates
- PMID: 38504766
- PMCID: PMC10947523
- DOI: 10.1002/ange.202117524
NHC Catalysis for Umpolung Pyridinium Alkylation via Deoxy-Breslow Intermediates
Abstract
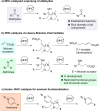
Umpolung N-heterocyclic carbene (NHC) catalysis of non-aldehyde substrates offers new pathways for C-C bond formation, but has proven challenging to develop in terms of viable substrate classes. Here, we demonstrate that pyridinium ions can undergo NHC addition and subsequent intramolecular C-C bond formation through a deoxy-Breslow intermediate. The alkylation demonstrates, for the first time, that deoxy-Breslow intermediates are viable for catalytic umpolung of areniums.
NHC deoxy‐Breslow catalysis offers new umpolung possibilities for electron‐poor arene rings. NHC organocatalysis is largely restricted to aldehydes, with other electrophiles proving difficult to harness. It is shown that a pyridinium system can react successfully with an NHC, enabling intramolecular C−C bond formation with a Michael acceptor through a deoxy‐Breslow intermediate.
Keywords: Deoxy-Breslow Intermediate; N-Heterocyclic Carbenes; Organocatalysis; Pyridinium Substrates; Umpolung.
© 2022 The Authors. Angewandte Chemie published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- None
-
- Breslow R., J. Am. Chem. Soc. 1958, 80, 3719–3726;
-
- Seebach D., Angew. Chem. Int. Ed. Engl. 1979, 18, 239–258;
- Angew. Chem. 1979, 91, 259–278.
-
- Reviews:
-
- Enders D., Niemeier O., Henseler A., Chem. Rev. 2007, 107, 5606–5655; - PubMed
LinkOut - more resources
Full Text Sources
