A phase I clinical trial assessing the safety, tolerability, and pharmacokinetics of inhaled ethanol in humans as a potential treatment for respiratory tract infections
- PMID: 38504921
- PMCID: PMC10949138
- DOI: 10.3389/fmed.2024.1324686
A phase I clinical trial assessing the safety, tolerability, and pharmacokinetics of inhaled ethanol in humans as a potential treatment for respiratory tract infections
Abstract
Background: Current treatments for respiratory infections are severely limited. Ethanol's unique properties including antimicrobial, immunomodulatory, and surfactant-like activity make it a promising candidate treatment for respiratory infections if it can be delivered safely to the airway by inhalation. Here, we explore the safety, tolerability, and pharmacokinetics of inhaled ethanol in a phase I clinical trial.
Methods: The study was conducted as a single-centre, open-label clinical trial in 18 healthy adult volunteers, six with no significant medical comorbidities, four with stable asthma, four with stable cystic fibrosis, and four active smokers. A dose-escalating design was used, with participants receiving three dosing cycles of 40, 60%, and then 80% ethanol v/v in water, 2 h apart, in a single visit. Ethanol was nebulised using a standard jet nebuliser, delivered through a novel closed-circuit reservoir system, and inhaled nasally for 10 min, then orally for 30 min. Safety assessments included adverse events and vital sign monitoring, blood alcohol concentrations, clinical examination, spirometry, electrocardiogram, and blood tests.
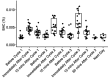
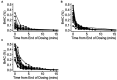
Results: No serious adverse events were recorded. The maximum blood alcohol concentration observed was 0.011% immediately following 80% ethanol dosing. Breath alcohol concentrations were high (median 0.26%) following dosing suggesting high tissue levels were achieved. Small transient increases in heart rate, blood pressure, and blood neutrophil levels were observed, with these normalising after dosing, with no other significant safety concerns. Of 18 participants, 15 completed all dosing cycles with three not completing all cycles due to tolerability. The closed-circuit reservoir system significantly reduced fugitive aerosol loss during dosing.
Conclusion: These data support the safety of inhaled ethanol at concentrations up to 80%, supporting its further investigation as a treatment for respiratory infections.Clinical trial registration: identifier ACTRN12621000067875.
Keywords: clinical trial; ethanol; pharmacokinetics; safety; tolerability.
Copyright © 2024 Hancock, Ditcham, Ferguson, Karpievitch, Stick, Waterer and Clements.
Conflict of interest statement
BC is a director of Inspiring Pty Ltd. who are developing the reservoir spacer system for commercialisation. WD has shares in Inspiring and has been contracted by Inspiring to perform aerosol testing in the past. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

