Sonomechanobiology: Vibrational stimulation of cells and its therapeutic implications
- PMID: 38504927
- PMCID: PMC10903386
- DOI: 10.1063/5.0127122
Sonomechanobiology: Vibrational stimulation of cells and its therapeutic implications
Abstract
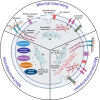
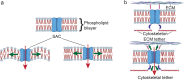
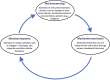
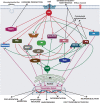
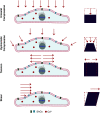
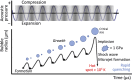
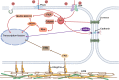
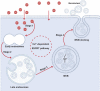
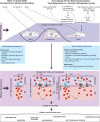
All cells possess an innate ability to respond to a range of mechanical stimuli through their complex internal machinery. This comprises various mechanosensory elements that detect these mechanical cues and diverse cytoskeletal structures that transmit the force to different parts of the cell, where they are transcribed into complex transcriptomic and signaling events that determine their response and fate. In contrast to static (or steady) mechanostimuli primarily involving constant-force loading such as compression, tension, and shear (or forces applied at very low oscillatory frequencies ( Hz) that essentially render their effects quasi-static), dynamic mechanostimuli comprising more complex vibrational forms (e.g., time-dependent, i.e., periodic, forcing) at higher frequencies are less well understood in comparison. We review the mechanotransductive processes associated with such acoustic forcing, typically at ultrasonic frequencies ( kHz), and discuss the various applications that arise from the cellular responses that are generated, particularly for regenerative therapeutics, such as exosome biogenesis, stem cell differentiation, and endothelial barrier modulation. Finally, we offer perspectives on the possible existence of a universal mechanism that is common across all forms of acoustically driven mechanostimuli that underscores the central role of the cell membrane as the key effector, and calcium as the dominant second messenger, in the mechanotransduction process.
© 2023 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
