Drug fever: a narrative review
- PMID: 38504950
- PMCID: PMC10944987
- DOI: 10.37737/ace.23013
Drug fever: a narrative review
Abstract
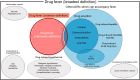
Drug fever is an adverse drug reaction accompanied by a febrile response and is a common problem among clinicians, hence an updated knowledge of drug fever is important. A consensus regarding the definition of drug fever is lacking. Thus, descriptions of drug fever in previous literature are often inconsistent. In this narrative review, we summarized various features of drug fever, including its definition, epidemiology, risk factors, clinical presentation, diagnosis, treatment and prognosis, based on the earliest literature. Recent advances in information technology have encouraged researchers to use pharmacovigilance databases for clinical and pharmacological research. We outlined how a pharmacovigilance database, along with recently developed research methods, could be used to research drug fever.
Keywords: drug adverse reactions; drug fever; narrative review.
© 2023 Society for Clinical Epidemiology.
Conflict of interest statement
The authors have nothing to declare.
Figures

References
-
- Musher DM, Fainstein V, Young EJ, Pruett TL. Fever patterns. Their lack of clinical significance. Arch Intern Med 1979;139:1225–1228. - PubMed
-
- Kurz A. Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol 2008;22:627–644. - PubMed
-
- Gell PGH, Coombs RRA, Others. Clinical aspects of immunology. Clinical Aspects of Immunology Published Online First: 1963. https://www.cabdirect.org/cabdirect/abstract/19632705010
-
- Lawley TJ, Bielory L, Gascon P, Yancey KB, Young NS, Frank MM. A prospective clinical and immunologic analysis of patients with serum sickness. N Engl J Med 1984;311:1407–1413. - PubMed
-
- Patel RA, Gallagher JC. Drug fever. Pharmacotherapy 2010;30:57–69. - PubMed
LinkOut - more resources
Full Text Sources
