Challenges and Breakthroughs in Selective Amide Activation
- PMID: 38504998
- PMCID: PMC10947092
- DOI: 10.1002/ange.202212213
Challenges and Breakthroughs in Selective Amide Activation
Abstract
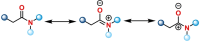
In contrast to ketones and carboxylic esters, amides are classically seen as comparatively unreactive members of the carbonyl family, owing to their unique structural and electronic features. However, recent decades have seen the emergence of research programmes focused on the selective activation of amides under mild conditions. In the past four years, this area has continued to rapidly develop, with new advances coming in at a fast pace. Several novel activation strategies have been demonstrated as effective tools for selective amide activation, enabling transformations that are at once synthetically useful and mechanistically intriguing. This Minireview comprises recent advances in the field, highlighting new trends and breakthroughs in what could be called a new age of amide activation.
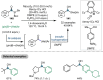
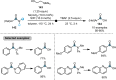
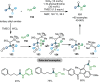
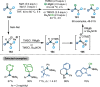
Research on the selective activation of amides has flourished over the last decades. In the past four years, this area has become a rapidly developing domain. This Minireview aims to highlight the breakthroughs in amide activation achieved since 2018, with a focus on significant advances in electrophilic and transition‐metal‐catalysed amide activation, as well as other strategies on amide activation and functionalisation.
Keywords: Amide Activation; Amide Functionalisation; Electrophilic Activation; Synthetic Methods; Transition-Metal Catalysis.
© 2022 The Authors. Angewandte Chemie published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














Similar articles
-
Challenges and Breakthroughs in Selective Amide Activation.Angew Chem Int Ed Engl. 2022 Dec 5;61(49):e202212213. doi: 10.1002/anie.202212213. Epub 2022 Nov 2. Angew Chem Int Ed Engl. 2022. PMID: 36124856 Free PMC article. Review.
-
Nucleophilic and Electrophilic Activation of Non-Heteroaromatic Amides in Atom-Economical Asymmetric Catalysis.Chemistry. 2016 Oct 17;22(43):15192-15200. doi: 10.1002/chem.201602484. Epub 2016 Aug 22. Chemistry. 2016. PMID: 27546485 Review.
-
Well-Defined Palladium(II)-NHC Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective N-C/O-C Cleavage.Acc Chem Res. 2018 Oct 16;51(10):2589-2599. doi: 10.1021/acs.accounts.8b00410. Epub 2018 Sep 21. Acc Chem Res. 2018. PMID: 30240190
-
Well-Defined Pre-Catalysts in Amide and Ester Bond Activation.Molecules. 2019 Jan 9;24(2):215. doi: 10.3390/molecules24020215. Molecules. 2019. PMID: 30634382 Free PMC article. Review.
-
Amide activation: an emerging tool for chemoselective synthesis.Chem Soc Rev. 2018 Oct 29;47(21):7899-7925. doi: 10.1039/c8cs00335a. Chem Soc Rev. 2018. PMID: 30152510 Review.
References
-
- Greenberg A., Breneman C. M., Liebman J. F., The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science, Wiley, New York, 2003.
-
- Bennet A. J., Wang Q. P., Slebocka-Tilk H., Somayaji V., Brown R. S., Santarsiero B. D., J. Am. Chem. Soc. 1990, 112, 6383–6385.
-
- Slebocka-Tilk H., Brown R. S., J. Org. Chem. 1987, 52, 805–808.
-
- Pauling L., The Nature of the Chemical Bond, Cornell University Press, Ithaca, 1960.
-
- Kaiser D., Bauer A., Lemmerer M., Maulide N., Chem. Soc. Rev. 2018, 47, 7899–7925. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
