Hatching plasticity is associated with a more advanced stage at hatching in an Ambystoma with terrestrial eggs
- PMID: 38505175
- PMCID: PMC10948370
- DOI: 10.1002/ece3.11160
Hatching plasticity is associated with a more advanced stage at hatching in an Ambystoma with terrestrial eggs
Abstract
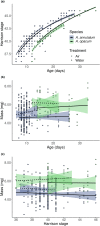
Hatching plasticity allows animals to initiate hatching in response to environmental cues including predation, flooding, and hypoxia. In species with terrestrial eggs but aquatic larvae, hatching plasticity often manifests as extended development of embryos when water is not available. Although these effects are taxonomically widespread, little attention has focused on differences in plasticity across closely related species with terrestrial and aquatic embryos. We propose that the terrestrial embryonic environment favors slower and prolonged development and, consequently, that we should see differences in development between closely related species that differ in where they lay their eggs. We test this hypothesis by comparing embryonic development between two mole salamanders, Ambystoma opacum and A. annulatum. Most Ambystoma lay eggs submerged in ponds but A. opacum lays its eggs on land, where hatching is triggered when eggs are submerged by rising pond levels. Embryos of both species were reared under common laboratory conditions simulating both aquatic and terrestrial nest sites. Consistent with our hypothesis, we found that A. opacum embryos exhibited slower development and took longer to hatch than A. annulatum embryos in both rearing environments. Furthermore, we observed in A. opacum a plasticity in hatching stage that was absent in A. annulatum. Our results indicate that the terrestrial-laying A. opacum has evolved slower and prolonged development relative to its aquatic-laying congener and suggest that embryonic survival in the unpredictable terrestrial environment may be facilitated by developmental plasticity.
Keywords: amphibian; embryo; extended development; hatching plasticity; larva.
© 2024 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
Neither author has a conflict of interest with respect to this project or in the publication of these data. Both authors contributed equally to designing and collecting data. REH analyzed the data and prepared figures and tables. KT prepared the first version of the manuscript for her undergraduate thesis, which was revised by REH for publication. This study was funded by a University of North Carolina System Interinstitutional Planning Grant to R. E. Hale and by the UNC Asheville Biology Department. The work was approved by the UNC Asheville Institutional Animal Care and Use Committee (Protocol 2019‐Res04). The authors have no competing interests to declare.
Figures





References
-
- Agresti, A. (1996). An introduction to categorical data analysis. In Barnet V., Bradley R. A., Fisher N. I., Hunter J. S., Kadane J. B., Kendall D. G., Scott D. W., Smith A. F. M., Teugels J. L., & Watson G. S. (Eds.), Wiley series in probability and statistics. John Wiley & Sons.
-
- Allen, J. D. , Schrage, K. R. , Foo, S. A. , Watson, S.‐A. , & Byrne, M. (2017). The effects of salinity and pH on fertilization, early development, and hatching in the grown‐of‐thorns seastar. Diversity, 9, 13. 10.3390/d9010013 - DOI
-
- Anderson, J. D. , & Williamson, G. K. (1976). Terrestrial mode of reproduction in Ambystoma cingulatum . Herpetologica, 32(2), 214–221.
-
- Bates, D. , Maechler, M. , Bolker, B. M. , & Walker, S. (2015). Fitting linear effects‐mixed models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 . - DOI
Associated data
LinkOut - more resources
Full Text Sources

