Structural, morphological, electrical, and dielectric properties of Na2Cu5(Si2O7)2 for ASSIBs
- PMID: 38505384
- PMCID: PMC10949118
- DOI: 10.1039/d4ra01454e
Structural, morphological, electrical, and dielectric properties of Na2Cu5(Si2O7)2 for ASSIBs
Retraction in
-
Retraction: Structural, morphological, electrical, and dielectric properties of Na2Cu5(Si2O7)2 for ASSIBs.RSC Adv. 2025 May 14;15(20):16097. doi: 10.1039/d5ra90057c. eCollection 2025 May 12. RSC Adv. 2025. PMID: 40370850 Free PMC article.
Expression of concern in
-
Expression of concern: Structural, morphological, electrical, and dielectric properties of Na2Cu5(Si2O7)2 for ASSIBs.RSC Adv. 2024 Dec 23;14(54):40179. doi: 10.1039/d4ra90155j. eCollection 2024 Dec 17. RSC Adv. 2024. PMID: 39720261 Free PMC article.
Abstract
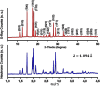
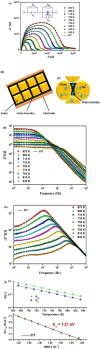
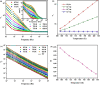
Solid inorganic electrolyte materials are fundamental components for constructing all-solid-state sodium-ion batteries. These solid electrolytes offer considerable benefits related to safety, electrochemical performance, and mechanical stability in comparison to liquid organic electrolyte systems. This study investigates the sodium ion conduction mechanism and relaxation kinetics in the sorosilicate material Na2Cu5(Si2O7)2, a potential solid electrolyte, using impedance spectroscopy. Analysis of the DC conductivity data demonstrates that sodium ion mobility follows Arrhenius behavior with a thermal activation energy barrier of 1.21 eV. This work highlights the importance of carefully choosing an appropriate equivalent circuit model to extract DC conductivity parameters from impedance data, given the contributions from both grain and grain boundary effects. Analysis of the AC conductivity and dielectric constant as a function of frequency and temperature demonstrates that ionic conduction takes place in this material through a process in which charge carriers overcome correlated energy barriers, known as correlated barrier hopping. The neutron diffraction patterns were analyzed using soft bond valence sum (BVS) techniques to map the possible ionic conduction pathways within the unit cell. Examination of the data points to obstructions in the sodium ion diffusion routes along the a-axis and diagonal of the bc plane within the triclinic unit cell. These bottlenecks likely contribute to the high activation energy and correspondingly low ionic conductivity observed. Analysis of dielectric properties by modulus verified that the ionic conduction relaxation phenomena exhibit thermal activation and a distribution of relaxation times. In summary, this work elucidates the microscopic ionic conduction mechanism in Na2Cu5(Si2O7)2 through extensive analysis encompassing DC/AC conductivity, electric modulus, and dielectric constant measurements. The insights gained into the ionic conduction mechanism will aid in engineering optimized ionic conductor materials for battery technologies.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Abraham K. M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020;5(11):3544–3547. doi: 10.1021/acsenergylett.0c02181. - DOI
-
- Fegade U. Jethave G. Khan F. Al-Ahmed A. Karmouch R. Shariq M. Inamuddin Ahmer M. F. Recent Development of Aqueous Zinc-ion Battery Cathodes and Future Challenges: Review. Int. J. Energy Res. 2022;46(10):13152–13177. doi: 10.1002/er.8018. - DOI
-
- Alzharani A. A. Shehab M. K. Rodene D. Ahmed J. U. Bakry A. M. Kaid M. M. El-Kaderi H. M. Surface Modification of Partially Reduced Graphene Oxide for Advanced Electrode Material in Rechargeable Sodium Batteries. Energy Fuels. 2022;36(9):4967–4977. doi: 10.1021/acs.energyfuels.2c00193. - DOI
Publication types
LinkOut - more resources
Full Text Sources

