Contact-Heat Evoked Potentials: Insights into Pain Processing in CRPS Type I
- PMID: 38505501
- PMCID: PMC10949273
- DOI: 10.2147/JPR.S436645
Contact-Heat Evoked Potentials: Insights into Pain Processing in CRPS Type I
Abstract
Purpose: The pathophysiological mechanisms underlying the development of chronic pain in complex regional pain syndrome (CRPS) are diverse and involve both peripheral and central changes in pain processing, such as sensitization of the nociceptive system. The aim of this study was to objectively distinguish the specific changes occurring at both peripheral and central levels in nociceptive processing in individuals with chronic CRPS type I.
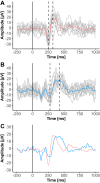
Patients and methods: Nineteen individuals with chronic CRPS type I and 16 age- and sex-matched healthy controls (HC) were recruited. All individuals underwent a clinical examination and pain assessment in the most painful limb, the contralateral limb, and a pain-free control area to distinguish between peripheral and central mechanisms. Contact-heat evoked potentials (CHEPs) were recorded after heat stimulation of the three different areas and amplitudes and latencies were analyzed. Additionally, quantitative sensory testing (QST) was performed in all three areas.
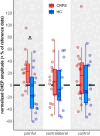
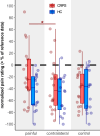
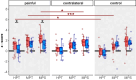
Results: Compared to HC, CHEP amplitudes in CRPS were only increased after stimulation of the painful area (p=0.025), while no increases were observed for the pain-free control area (p=0.14). None of the CHEP latencies were different between the two cohorts (all p>0.23). Furthermore, individuals with CRPS showed higher pain ratings after stimulation of the painful limb compared to their contralateral limb (p=0.013). Lastly, compared to HC, mechanical (p=0.012) and thermal (p=0.046) sensitivity was higher in the painful area of the CRPS cohort.
Conclusion: This study provides neurophysiological evidence supporting an intact thermo-nociceptive pathway with signs of peripheral sensitization, such as hyperexcitable primary afferent nociceptors, in individuals with CRPS type I. This is further supported by the observation of mechanical and thermal gain of sensation only in the painful limb. Additionally, the increased CHEP amplitudes might be related to fear-induced alterations of nociceptive processing.
Keywords: complex regional pain syndrome; contact-heat evoked potentials; pain hypersensitivity; pain mechanism; sensitization; thermo-nociceptive processing.
© 2024 Allmendinger et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures