Loss of the auxiliary α2δ1 voltage-sensitive calcium channel subunit impairs bone formation and anabolic responses to mechanical loading
- PMID: 38505532
- PMCID: PMC10945727
- DOI: 10.1093/jbmrpl/ziad008
Loss of the auxiliary α2δ1 voltage-sensitive calcium channel subunit impairs bone formation and anabolic responses to mechanical loading
Abstract
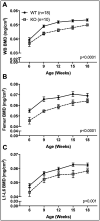
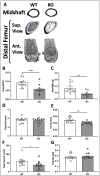
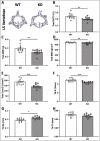
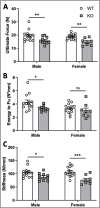
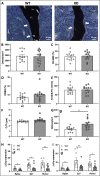
Voltage-sensitive calcium channels (VSCCs) influence bone structure and function, including anabolic responses to mechanical loading. While the pore-forming (α1) subunit of VSCCs allows Ca2+ influx, auxiliary subunits regulate the biophysical properties of the pore. The α2δ1 subunit influences gating kinetics of the α1 pore and enables mechanically induced signaling in osteocytes; however, the skeletal function of α2δ1 in vivo remains unknown. In this work, we examined the skeletal consequences of deleting Cacna2d1, the gene encoding α2δ1. Dual-energy X-ray absorptiometry and microcomputed tomography imaging demonstrated that deletion of α2δ1 diminished bone mineral content and density in both male and female C57BL/6 mice. Structural differences manifested in both trabecular and cortical bone for males, while the absence of α2δ1 affected only cortical bone in female mice. Deletion of α2δ1 impaired skeletal mechanical properties in both sexes, as measured by three-point bending to failure. While no changes in osteoblast number or activity were found for either sex, male mice displayed a significant increase in osteoclast number, accompanied by increased eroded bone surface and upregulation of genes that regulate osteoclast differentiation. Deletion of α2δ1 also rendered the skeleton insensitive to exogenous mechanical loading in males. While previous work demonstrates that VSCCs are essential for anabolic responses to mechanical loading, the mechanism by which these channels sense and respond to force remained unclear. Our data demonstrate that the α2δ1 auxiliary VSCC subunit functions to maintain baseline bone mass and strength through regulation of osteoclast activity and also provides skeletal mechanotransduction in male mice. These data reveal a molecular player in our understanding of the mechanisms by which VSCCs influence skeletal adaptation.
Keywords: bone formation; loading; mechanotransduction; voltage-sensitive calcium channel; α2δ1 subunit.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
None declared.
Figures




Similar articles
-
Deletion of the auxiliary α2δ1 voltage sensitive calcium channel subunit in osteocytes and late-stage osteoblasts impairs femur strength and load-induced bone formation in male mice.J Bone Miner Res. 2024 Apr 19;39(3):298-314. doi: 10.1093/jbmr/zjae010. J Bone Miner Res. 2024. PMID: 38477790
-
Gabapentin Disrupts Binding of Perlecan to the α2δ1 Voltage Sensitive Calcium Channel Subunit and Impairs Skeletal Mechanosensation.Biomolecules. 2022 Dec 12;12(12):1857. doi: 10.3390/biom12121857. Biomolecules. 2022. PMID: 36551284 Free PMC article.
-
Skeletal Functions of Voltage Sensitive Calcium Channels.Curr Osteoporos Rep. 2021 Apr;19(2):206-221. doi: 10.1007/s11914-020-00647-7. Epub 2021 Mar 15. Curr Osteoporos Rep. 2021. PMID: 33721180 Free PMC article. Review.
-
Association of the α(2)δ(1) subunit with Ca(v)3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes.J Bone Miner Res. 2011 Sep;26(9):2125-39. doi: 10.1002/jbmr.437. J Bone Miner Res. 2011. PMID: 21638318 Free PMC article.
-
Examining Mechanisms for Voltage-Sensitive Calcium Channel-Mediated Secretion Events in Bone Cells.Calcif Tissue Int. 2023 Jul;113(1):126-142. doi: 10.1007/s00223-023-01097-w. Epub 2023 Jun 1. Calcif Tissue Int. 2023. PMID: 37261463 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

