FSH induces EMT in ovarian cancer via ALKBH5-regulated Snail m6A demethylation
- PMID: 38505602
- PMCID: PMC10945345
- DOI: 10.7150/thno.94161
FSH induces EMT in ovarian cancer via ALKBH5-regulated Snail m6A demethylation
Abstract
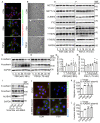
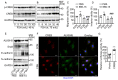
Background: The therapeutic benefits of targeting follicle-stimulating hormone (FSH) receptor in treatment of ovarian cancer are significant, whereas the role of FSH in ovarian cancer progresses and the underlying mechanism remains to be developed. Methods: Tissue microarray of human ovarian cancer, tumor xenograft mouse model, and in vitro cell culture were used to investigate the role of FSH in ovarian carcinogenesis. siRNA, lentivirus and inhibitors were used to trigger the inactivation of genes, and plasmids were used to increase transcription of genes. Specifically, pathological characteristic was assessed by histology and immunohistochemistry (IHC), while signaling pathway was studied using western blot, quantitative RT-PCR, and immunofluorescence. Results: Histology and IHC of human normal ovarian and tumor tissue confirmed the association between FSH and Snail in ovarian cancer metastasis. Moreover, in epithelial ovarian cancer cells and xenograft mice, FSH was showed to promote epithelial mesenchymal transition (EMT) progress and metastasis of ovarian cancer via prolonging the half-life of Snail mRNA in a N6-methyladenine methylation (m6A) dependent manner, which was mechanistically through the CREB/ALKBH5 signaling pathway. Conclusions: These findings indicated that FSH induces EMT progression and ovarian cancer metastasis via CREB/ALKBH5/Snail pathway. Thus, this study provided new insight into the therapeutic strategy of ovarian cancer patients with high level of FSH.
Keywords: ALKBH5; Epithelial ovarian cancer; Epithelial-mesenchymal transition; Follicle-stimulating hormone; Snail.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. - PubMed
-
- Veneziani AC, Gonzalez-Ochoa E, Alqaisi H, Madariaga A, Bhat G, Rouzbahman M. et al. Heterogeneity and treatment landscape of ovarian carcinoma. Nat Rev Clin Oncol. 2023;20:820–42. - PubMed
-
- Konishi I, Kuroda H, Mandai M. Review: Gonadotropins and development of ovarian cancer. Oncology. 1999;57(Suppl 2):45–8. - PubMed
-
- Choi KC, Kang SK, Tai CJ, Auersperg N, Leung PC. Follicle-stimulating hormone activates mitogen-activated protein kinase in preneoplastic and neoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2002;87:2245–53. - PubMed
-
- Choi JH, Choi KC, Auersperg N, Leung PC. Gonadotropins upregulate the epidermal growth factor receptor through activation of mitogen-activated protein kinases and phosphatidyl-inositol-3-kinase in human ovarian surface epithelial cells. Endocr Relat Cancer. 2005;12:407–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

