A Communication and Decision-Making Framework for Pediatric Precision Medicine
- PMID: 38505927
- PMCID: PMC10979296
- DOI: 10.1542/peds.2023-062850
A Communication and Decision-Making Framework for Pediatric Precision Medicine
Abstract
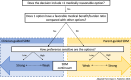
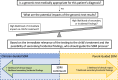
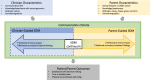
Advances in genomic testing have been pivotal in moving childhood cancer care forward, with genomic testing now a standard diagnostic tool for many children, adolescents, and young adults with cancer. Beyond oncology, the role of genomic testing in pediatric research and clinical care is growing, including for children with developmental differences, cardiac abnormalities, and epilepsy. Despite more standard use in their patients, pediatricians have limited guidance on how to communicate this complex information or how to engage parents in decisions related to precision medicine. Drawing from empirical work in pediatric informed consent and existing models of shared decision-making, we use pediatric precision cancer medicine as a case study to propose a conceptual framework to approach communication and decision-making about genomic testing in pediatrics. The framework relies on identifying the type of genomic testing, its intended role, and its anticipated implications to inform the scope of information delivered and the parents' role in decision-making (leading to shared decision-making along a continuum from clinician-guided to parent-guided). This type of framework rests on practices known to be standard in other complex decision-making but also integrates unique features of genomic testing and precision medicine. With the increasing prominence of genomics and precision medicine in pediatrics, with our communication and decision-making framework, we aim to guide clinicians to better support their pediatric patients and their parents in making informed, goal-concordant decisions throughout their care trajectory.
Copyright © 2024 by the American Academy of Pediatrics.
Conflict of interest statement
Figures
References
-
- Trautmann M, Hartmann W. Molecular approaches to diagnosis in Ewing sarcoma: fluorescence in situ hybridization (FISH). Methods Mol Biol. 2021;2226:65–83 - PubMed
-
- Hiemenz MC, Oberley MJ, Doan A, et al. . A multimodal genomics approach to diagnostic evaluation of pediatric hematologic malignancies. Cancer Genet. 2021;254–255:25–33 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

