AKAP12 Upregulation Associates With PDE8A to Accelerate Cardiac Dysfunction
- PMID: 38506047
- PMCID: PMC11075752
- DOI: 10.1161/CIRCRESAHA.123.323655
AKAP12 Upregulation Associates With PDE8A to Accelerate Cardiac Dysfunction
Abstract
Background: In heart failure, signaling downstream the β2-adrenergic receptor is critical. Sympathetic stimulation of β2-adrenergic receptor alters cAMP (cyclic adenosine 3',5'-monophosphate) and triggers PKA (protein kinase A)-dependent phosphorylation of proteins that regulate cardiac function. cAMP levels are regulated in part by PDEs (phosphodiesterases). Several AKAPs (A kinase anchoring proteins) regulate cardiac function and are proposed as targets for precise pharmacology. AKAP12 is expressed in the heart and has been reported to directly bind β2-adrenergic receptor, PKA, and PDE4D. However, its roles in cardiac function are unclear.
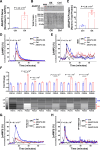
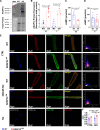
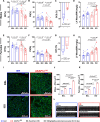
Methods: cAMP accumulation in real time downstream of the β2-adrenergic receptor was detected for 60 minutes in live cells using the luciferase-based biosensor (GloSensor) in AC16 human-derived cardiomyocyte cell lines overexpressing AKAP12 versus controls. Cardiomyocyte intracellular calcium and contractility were studied in adult primary cardiomyocytes from male and female mice overexpressing cardiac AKAP12 (AKAP12OX) and wild-type littermates post acute treatment with 100-nM isoproterenol (ISO). Systolic cardiac function was assessed in mice after 14 days of subcutaneous ISO administration (60 mg/kg per day). AKAP12 gene and protein expression levels were evaluated in left ventricular samples from patients with end-stage heart failure.
Results: AKAP12 upregulation significantly reduced total intracellular cAMP levels in AC16 cells through PDE8. Adult primary cardiomyocytes from AKAP12OX mice had significantly reduced contractility and impaired calcium handling in response to ISO, which was reversed in the presence of the selective PDE8 inhibitor (PF-04957325). AKAP12OX mice had deteriorated systolic cardiac function and enlarged left ventricles. Patients with end-stage heart failure had upregulated gene and protein levels of AKAP12.
Conclusions: AKAP12 upregulation in cardiac tissue is associated with accelerated cardiac dysfunction through the AKAP12-PDE8 axis.
Keywords: A kinase anchor proteins; calcium; echocardiography; heart; myocytes, cardiac; receptors, adrenergic, beta.
Conflict of interest statement
Figures





Comment in
-
AKAP12 Overexpression Affects Cardiac Function via PDE8.Circ Res. 2024 Apr 12;134(8):1023-1025. doi: 10.1161/CIRCRESAHA.124.324475. Epub 2024 Apr 11. Circ Res. 2024. PMID: 38603476 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

