Combining VPS34 inhibitors with STING agonists enhances type I interferon signaling and anti-tumor efficacy
- PMID: 38506049
- PMCID: PMC11306511
- DOI: 10.1002/1878-0261.13619
Combining VPS34 inhibitors with STING agonists enhances type I interferon signaling and anti-tumor efficacy
Abstract
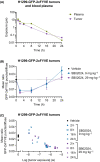
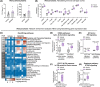
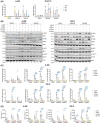
An immunosuppressive tumor microenvironment promotes tumor growth and is one of the main factors limiting the response to cancer immunotherapy. We have previously reported that inhibition of vacuolar protein sorting 34 (VPS34), a crucial lipid kinase in the autophagy/endosomal trafficking pathway, decreases tumor growth in several cancer models, increases infiltration of immune cells and sensitizes tumors to anti-programmed cell death protein 1/programmed cell death 1 ligand 1 therapy by upregulation of C-C motif chemokine 5 (CCL5) and C-X-C motif chemokine 10 (CXCL10) chemokines. The purpose of this study was to investigate the signaling mechanism leading to the VPS34-dependent chemokine increase. NanoString gene expression analysis was applied to tumors from mice treated with the VPS34 inhibitor SB02024 to identify key pathways involved in the anti-tumor response. We showed that VPS34 inhibitors increased the secretion of T-cell-recruitment chemokines in a cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes protein (STING)-dependent manner in cancer cells. Both pharmacological and small interfering RNA (siRNA)-mediated VPS34 inhibition increased cGAS/STING-mediated expression and secretion of CCL5 and CXCL10. The combination of VPS34 inhibitor and STING agonist further induced cytokine release in both human and murine cancer cells as well as monocytic or dendritic innate immune cells. Finally, the VPS34 inhibitor SB02024 sensitized B16-F10 tumor-bearing mice to STING agonist treatment and significantly improved mice survival. These results show that VPS34 inhibition augments the cGAS/STING pathway, leading to greater tumor control through immune-mediated mechanisms. We propose that pharmacological VPS34 inhibition may synergize with emerging therapies targeting the cGAS/STING pathway.
Keywords: STING; autophagy; cancer; immunotherapy; interferons.
© 2024 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
YY, SP, LT, CS, JL, JV, MA, JM, and ADM are employees/shareholders of Sprint Bioscience. MB, JS, MJT, HA, BDS, and DLF are employees/shareholders of Deciphera Pharmaceuticals. The other authors declare no potential conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

