Catalyst and Medium Control over Rebound Pathways in Manganese-Catalyzed Methylenic C-H Bond Oxidation
- PMID: 38506665
- PMCID: PMC10996012
- DOI: 10.1021/jacs.3c11555
Catalyst and Medium Control over Rebound Pathways in Manganese-Catalyzed Methylenic C-H Bond Oxidation
Abstract
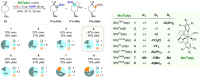
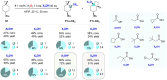
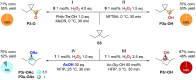
The C(sp3)-H bond oxygenation of a variety of cyclopropane containing hydrocarbons with hydrogen peroxide catalyzed by manganese complexes containing aminopyridine tetradentate ligands was carried out. Oxidations were performed in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) and 2,2,2-trifluoroethanol (TFE) using different manganese catalysts and carboxylic acid co-ligands, where steric and electronic properties were systematically modified. Functionalization selectively occurs at the most activated C-H bonds that are α- to cyclopropane, providing access to carboxylate or 2,2,2-trifluoroethanolate transfer products, with no competition, in favorable cases, from the generally dominant hydroxylation reaction. The formation of mixtures of unrearranged and rearranged esters (oxidation in HFIP in the presence of a carboxylic acid) and ethers (oxidation in TFE) with full control over diastereoselectivity was observed, confirming the involvement of delocalized cationic intermediates in these transformations. Despite such a complex mechanistic scenario, by fine-tuning of catalyst and carboxylic acid sterics and electronics and leveraging on the relative contribution of cationic pathways to the reaction mechanism, control over product chemoselectivity could be systematically achieved. Taken together, the results reported herein provide powerful catalytic tools to rationally manipulate ligand transfer pathways in C-H oxidations of cyclopropane containing hydrocarbons, delivering novel products in good yields and, in some cases, outstanding selectivities, expanding the available toolbox for the development of synthetically useful C-H functionalization procedures.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Chambers R. K.; Weaver J. D.; Kim J.; Hoar J. L.; Krska S. W.; White M. C. A preparative small-molecule mimic of liver CYP450 enzymes in the aliphatic C–H oxidation of carbocyclic N-heterocycles. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2300315120.310.1073/pnas.2300315120. - DOI - PMC - PubMed
- Santana V. C. S.; Fernandes M. C. V.; Cappuccelli I.; Richieri A. C. G.; de Lucca E. C. Jr. Metal-Catalyzed C–H Bond Oxidation in the Total Synthesis of Natural and Unnatural Products. Synthesis 2022, 54, 5337–5359. 10.1055/a-1918-4338. - DOI
- Genovino J.; Sames D.; Hamann L. G.; Touré B. B. Accessing Drug Metabolites via Transition-Metal Catalyzed C–H Oxidation: The Liver as Synthetic Inspiration. Angew. Chem., Int. Ed. 2016, 55, 14218–14238. 10.1002/anie.201602644. - DOI - PubMed
-
- Bryliakov K. P. Transition Metal-Catalyzed Direct Stereoselective Oxygenations of C(sp3)–H Groups. ACS Catal. 2023, 13, 10770–10795. 10.1021/acscatal.3c02282. - DOI
- Chen J.; Song W.; Yao J.; Wu Z.; Lee Y.-M.; Wang Y.; Nam W.; Wang B. Hydrogen Bonding-Assisted and Nonheme Manganese-Catalyzed Remote Hydroxylation of C–H Bonds in Nitrogen-Containing Molecules. J. Am. Chem. Soc. 2023, 145, 5456–5466. 10.1021/jacs.2c13832. - DOI - PubMed
- White M. C.; Zhao J. Aliphatic C–H Oxidations for Late-Stage Functionalization. J. Am. Chem. Soc. 2018, 140, 13988–14009. 10.1021/jacs.8b05195. - DOI - PMC - PubMed
- Milan M.; Salamone M.; Costas M.; Bietti M. The Quest for Selectivity in Hydrogen Atom Transfer Based Aliphatic C–H Bond Oxygenation. Acc. Chem. Res. 2018, 51, 1984–1995. 10.1021/acs.accounts.8b00231. - DOI - PubMed
- Newhouse T.; Baran P. S. If C–H Bonds Could Talk: Selective C–H Bond Oxidation. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. 10.1002/anie.201006368. - DOI - PMC - PubMed
-
- Lee J. L.; Ross D. L.; Barman S. K.; Ziller J. W.; Borovik A. S. C–H Bond Cleavage by Bioinspired Nonheme Metal Complexes. Inorg. Chem. 2021, 60, 13759–13783. 10.1021/acs.inorgchem.1c01754. - DOI - PMC - PubMed
- Chen J.; Jiang Z.; Fukuzumi S.; Nam W.; Wang B. Artificial nonheme iron and manganese oxygenases for enantioselective olefin epoxidation and alkane hydroxylation reactions. Coord. Chem. Rev. 2020, 421, 21344310.1016/j.ccr.2020.213443. - DOI
- Vicens L.; Olivo G.; Costas M. Rational Design of Bioinspired Catalysts for Selective Oxidations. ACS Catal. 2020, 10, 8611–8631. 10.1021/acscatal.0c02073. - DOI
- Sun W.; Sun Q. Bioinspired Manganese and Iron Complexes for Enantioselective Oxidation Reactions: Ligand Design, Catalytic Activity, and Beyond. Acc. Chem. Res. 2019, 52, 2370–2381. 10.1021/acs.accounts.9b00285. - DOI - PubMed
- Que L.; Tolman W. B. Biologically inspired oxidation catalysis. Nature 2008, 455, 333–340. 10.1038/nature07371. - DOI - PubMed
-
- Huang X.; Groves J. T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. 10.1021/acs.chemrev.7b00373. - DOI - PMC - PubMed
- Huang X.; Groves J. T. Beyond ferryl-mediated hydroxylation: 40 years of the rebound mechanism and C–H activation. J. Biol. Inorg. Chem. 2017, 22, 185–207. 10.1007/s00775-016-1414-3. - DOI - PMC - PubMed
- Liu W.; Huang X.; Cheng M.-J.; Nielsen R. J.; Goddard W. A. III; Groves J. T. Oxidative Aliphatic C-H Fluorination with Fluoride Ion Catalyzed by a Manganese Porphyrin. Science 2012, 337, 1322–1325. 10.1126/science.1222327. - DOI - PubMed
- Liu W.; Groves J. T. Manganese Porphyrins Catalyze Selective C-H Bond Halogenations. J. Am. Chem. Soc. 2010, 132, 12847–12849. 10.1021/ja105548x. - DOI - PubMed
-
- Kal S.; Que L. Dioxygen activation by nonheme iron enzymes with the 2-His-1-carboxylate facial triad that generate high-valent oxoiron oxidants. J. Biol. Inorg. Chem. 2017, 22, 339–365. 10.1007/s00775-016-1431-2. - DOI - PubMed
- Koehntop K. D.; Emerson J. P.; Que L. Jr. The 2-His-1-carboxylate facial triad: a versatile platform for dioxygen activation by mononuclear non-heme iron(II) enzymes. J. Biol. Inorg. Chem. 2005, 10, 87–93. 10.1007/s00775-005-0624-x. - DOI - PubMed
- Costas M.; Mehn M. P.; Jensen M. P.; Que L. Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem. Rev. 2004, 104, 939–986. 10.1021/cr020628n. - DOI - PubMed
- Chen K.; Que L. Jr. Stereospecific Alkane Hydroxylation by Non-Heme Iron Catalysts: Mechanistic Evidence for an FeV=O Active Species. J. Am. Chem. Soc. 2001, 123, 6327–6337. 10.1021/ja010310x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

