Comparison of two self-expanding transcatheter heart valves for degenerated surgical bioprostheses: the AVENGER multicentre registry
- PMID: 38506737
- PMCID: PMC10941672
- DOI: 10.4244/EIJ-D-23-00779
Comparison of two self-expanding transcatheter heart valves for degenerated surgical bioprostheses: the AVENGER multicentre registry
Abstract
Background: There is a lack of comparative data on transcatheter aortic valve implantation (TAVI) in degenerated surgical prostheses (valve-in-valve [ViV]).
Aims: We sought to compare outcomes of using two self-expanding transcatheter heart valve (THV) systems for ViV.
Methods: In this retrospective multicentre registry, we included consecutive patients undergoing transfemoral ViV using either the ACURATE neo/neo2 (ACURATE group) or the Evolut R/PRO/PRO+ (EVOLUT group). The primary outcome measure was technical success according to Valve Academic Research Consortium (VARC)-3. Secondary outcomes were 30-day all-cause mortality, device success (VARC-3), coronary obstruction (CO) requiring intervention, rates of severe prosthesis-patient mismatch (PPM), and aortic regurgitation (AR) ≥moderate. Comparisons were made after 1:1 propensity score matching.
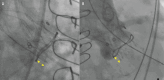
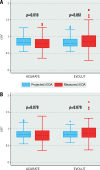
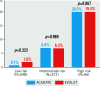
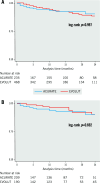
Results: The study cohort comprised 835 patients from 20 centres (ACURATE n=251; EVOLUT n=584). In the matched cohort (n=468), technical success (ACURATE 92.7% vs EVOLUT 88.9%; p=0.20) and device success (69.7% vs 73.9%; p=0.36) as well as 30-day mortality (2.8% vs 1.6%; p=0.392) were similar between the two groups. The mean gradients and rates of severe PPM, AR ≥moderate, or CO did not differ between the groups. Technical and device success were higher for the ACURATE platform among patients with a true inner diameter (ID) >19 mm, whereas a true ID ≤19 mm was associated with higher device success - but not technical success - among Evolut recipients.
Conclusions: ViV TAVI using either ACURATE or Evolut THVs showed similar procedural outcomes. However, a true ID >19 mm was associated with higher device success among ACURATE recipients, whereas in patients with a true ID ≤19 mm, device success was higher when using Evolut.
Conflict of interest statement
W. Kim reports personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Meril Life Sciences, Shockwave Medical, and HiD Imaging. M. Seiffert received speaker or advisory fees from Abbott, Abiomed, Amgen, AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Daichii Sankyo, Edwards Lifesciences, Inari Medical, Medtronic, Pfizer, Shockwave Medical, and Siemens Healthineers; and a research grant from Boston Scientific - all unrelated to the submitted work. A. Rück has received grants from Boston Scientific; and personal fees from Boston Scientific and Edwards Lifesciences. D. Leistner reports personal fees and non-financial support from Abbott; personal fees from Boston Scientific; and grants from DZHK (German Center for Cardiovascular Research). H. Dreger is on the advisory board and received speaker fees from Abbott and Edwards Lifesciences; and is a proctor for and received research funding from Abbott. M. Adam reports personal fees/speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic. H Möllmann reports proctor fees and/or speaker honoraria from Abbott, Biotronik, Edwards Lifesciences, Medtronic, SMT, and Boston Scientific. J. Blumenstein reports proctor fees from Boston Scientific. A. Holzamer reports proctoring for Boston Scientific. M. Barbanti is a consultant for Medtronic, Edwards Lifesciences, and Boston Scientific. C. Tamburino is a consultant for Medtronic. F. Castriota is a proctor for Boston Scientific, Medtronic, Abbott, and Terumo. R. Nerla is a proctor for Medtronic. C. Frerker received travel support and lecture honoraria from Medtronic and Boston Scientific. T. Schmidt received travel support and lecture honoraria from Medtronic and Boston Scientific. A. Wolf is a proctor for Medtronic, Boston Scientific, and Edwards Lifesciences. S. Toggweiler has served as a consultant for Medtronic, Boston Scientific, Edwards Lifesciences, Medira, Shockwave Medical, Teleflex, AtHeart Medical, Veosource, Polares Medical, and Biosensors; has served as a proctor for Medtronic, Boston Scientific, Edwards Lifesciences, Biosensors, and Abbott; has received institutional research grants from Boston Scientific, Biosensors, Fumedica, and Novartis; and holds equity in Hi-D Imaging. A. Mangieri received speaker honoraria from Boston Scientific and Abbott; received fees and speaker honoraria from Concept Medical; has received institutional grants from Boston Scientific, Abbott, and Kardia; and serves as proctor for Kardia. G. Kaleschke received speaker fees and travel grants from Edwards Lifesciences. E. Charitos reports proctor fees from Boston Scientific. M. Joner reports institutional grant support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and Infraredx; consulting fees from Biotronik, TriCares, Veryan, and Shockwave Medical; speaker fees from Abbott, AstraZeneca, Biotronik, Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, ReCor Medical, and Shockwave Medical; reports participation on the steering committees of Biotronik and Edwards Lifesciences; travel support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and SIS Medical. M. Vanhaverbeke is a consultant for Boston Scientific. M. Renker received proctor fees from Boston Scientific. T. Rheude received lecture fees from Abbott, AstraZeneca, SIS Medical AG and Translumina. The other authors have no conflicts of interest to declare.
Figures


Similar articles
-
Transcatheter Aortic Valve Replacement With Next-Generation Self-Expanding Devices: A Multicenter, Retrospective, Propensity-Matched Comparison of Evolut PRO Versus Acurate neo Transcatheter Heart Valves.JACC Cardiovasc Interv. 2019 Mar 11;12(5):433-443. doi: 10.1016/j.jcin.2018.11.036. JACC Cardiovasc Interv. 2019. PMID: 30846081
-
Comparison of Self-Expanding Bioprostheses for Transcatheter Aortic Valve Replacement in Patients With Symptomatic Severe Aortic Stenosis: SCOPE 2 Randomized Clinical Trial.Circulation. 2020 Dec 22;142(25):2431-2442. doi: 10.1161/CIRCULATIONAHA.120.051547. Epub 2020 Oct 15. Circulation. 2020. PMID: 33054367 Clinical Trial.
-
Multicenter comparison of transcatheter aortic valve implantation with the self-expanding ACURATE neo2 versus Evolut PRO transcatheter heart valves.Clin Res Cardiol. 2024 Jan;113(1):38-47. doi: 10.1007/s00392-023-02194-4. Epub 2023 Apr 28. Clin Res Cardiol. 2024. PMID: 37115228 Free PMC article.
-
TAVI with the ACURATE neo transcatheter heart valve in special populations: A systematic review.Hellenic J Cardiol. 2022 Jul-Aug;66:67-71. doi: 10.1016/j.hjc.2022.04.005. Epub 2022 May 1. Hellenic J Cardiol. 2022. PMID: 35508295
-
Effectiveness of the new generation transcatheter aortic valve in the real life studies. Review and meta-analysis.Eur Rev Med Pharmacol Sci. 2019 Sep;23(18):8018-8027. doi: 10.26355/eurrev_201909_19018. Eur Rev Med Pharmacol Sci. 2019. PMID: 31599427 Review.
Cited by
-
Efficacy and Safety of ACURATE neo2 in Valve-in-Valve TAVI: A Prospective Single-Center Study.J Clin Med. 2025 Jul 2;14(13):4677. doi: 10.3390/jcm14134677. J Clin Med. 2025. PMID: 40649052 Free PMC article.
-
Acurate Neo2 for valve-in-valve treatment of degenerated 3F Enable sutureless bioprosthetic valve in nonagenarian patient: a case report.Eur Heart J Case Rep. 2025 Mar 11;9(3):ytaf073. doi: 10.1093/ehjcr/ytaf073. eCollection 2025 Mar. Eur Heart J Case Rep. 2025. PMID: 40070802 Free PMC article.
References
-
- Ribeiro HB, Rodés-Cabau J, Blanke P, Leipsic J, Kwan Park, Bapat V, Makkar R, Simonato M, Barbanti M, Schofer J, Bleiziffer S, Latib A, Hildick-Smith D, Presbitero P, Windecker S, Napodano M, Cerillo AG, Abdel-Wahab M, Tchetche D, Fiorina C, Sinning JM, Cohen MG, Guerrero ME, Whisenant B, Nietlispach F, Palma JH, Nombela-Franco L, de Weger, Kass M, Sandoli de, Lemos PA, Kornowski R, Webb J, Dvir D. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J. 2018;39:687–95. - PubMed
-
- Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodés-Cabau J, Treede H, Piazza N, Hildick-Smith D, Himbert D, Walther T, Hengstenberg C, Nissen H, Bekeredjian R, Presbitero P, Ferrari E, Segev A, de Weger, Windecker S, Moat NE, Napodano M, Wilbring M, Cerillo AG, Brecker S, Tchetche D, Lefèvre T, De Marco, Fiorina C, Petronio AS, Teles RC, Testa L, Laborde JC, Leon MB, Kornowski R Valve-in-Valve International Data Registry Investigators. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;12:162–70. - PubMed
-
- Bieliauskas G, Wong I, Bajoras V, Wang X, Kofoed KF, De Backer, Søndergaard L. Patient-Specific Implantation Technique to Obtain Neo-Commissural Alignment With Self-Expanding Transcatheter Aortic Valves. JACC Cardiovasc Interv. 2021;14:2097–108. - PubMed
-
- Holzamer A, Kim WK, Rück A, Sathananthan J, Keller L, Cosma J, Bauer T, Nef H, Amat-Santos IJ, Brinkert M, Husser O, Pellegrini C, Schofer J, Nerla R, Montorfano M, Giannini F, Stella P, Kuwata S, Hilker M, Castriota F, Ussia GP, Webb JG, Nietlispach F, Toggweiler S. Valve-in-Valve Implantation Using the ACURATE Neo in Degenerated Aortic Bioprostheses: An International Multicenter Analysis. JACC Cardiovasc Interv. 2019;12:2309–16. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

