Research Advances in Neuroblast Migration in Traumatic Brain Injury
- PMID: 38507029
- PMCID: PMC11415452
- DOI: 10.1007/s12035-024-04117-4
Research Advances in Neuroblast Migration in Traumatic Brain Injury
Abstract
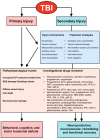
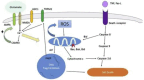
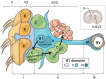
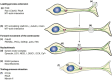
Neuroblasts were first derived from the adult mammalian brains in the 1990s by Reynolds et al. Since then, persistent neurogenesis in the subgranular zone (SGZ) of the hippocampus and subventricular zone (SVZ) has gradually been recognized. To date, reviews on neuroblast migration have largely investigated glial cells and molecular signaling mechanisms, while the relationship between vasculature and cell migration remains a mystery. Thus, this paper underlines the partial biological features of neuroblast migration and unravels the significance and mechanisms of the vasculature in the process to further clarify theoretically the neural repair mechanism after brain injury. Neuroblast migration presents three modes according to the characteristics of cells that act as scaffolds during the migration process: gliophilic migration, neurophilic migration, and vasophilic migration. Many signaling molecules, including brain-derived neurotrophic factor (BDNF), stromal cell-derived factor 1 (SDF-1), vascular endothelial growth factor (VEGF), and angiopoietin-1 (Ang-1), affect vasophilic migration, synergistically regulating the migration of neuroblasts to target areas along blood vessels. However, the precise role of blood vessels in the migration of neuroblasts needs to be further explored. The in-depth study of neuroblast migration will most probably provide theoretical basis and breakthrough for the clinical treatment of brain injury diseases.
Keywords: Neuroblast migration; Neurogenesis; Neuronal migration; Traumatic brain injury; Vascular migration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Guerrero-Cazares H, Lavell E, Chen L, Schiapparelli P, Lara-Velazquez M, Capilla-Gonzalez V, Clements AC, Drummond G et al (2017) Brief Report: Robo1 regulates the migration of human subventricular zone neural progenitor cells during development. Stem Cells 35:1860–1865. 10.1002/stem.2628 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

