Evaluation of mAb 2C5-modified dendrimer-based micelles for the co-delivery of siRNA and chemotherapeutic drug in xenograft mice model
- PMID: 38507033
- PMCID: PMC11208241
- DOI: 10.1007/s13346-024-01562-5
Evaluation of mAb 2C5-modified dendrimer-based micelles for the co-delivery of siRNA and chemotherapeutic drug in xenograft mice model
Erratum in
-
Correction: Evaluation of mAb 2C5-modified dendrimer-based micelles for the co-delivery of siRNA and chemotherapeutic drug in xenograft mice model.Drug Deliv Transl Res. 2024 Aug;14(8):2186-2187. doi: 10.1007/s13346-024-01601-1. Drug Deliv Transl Res. 2024. PMID: 38637432 Free PMC article. No abstract available.
Abstract
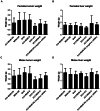
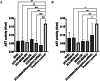
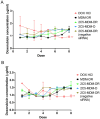
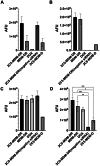
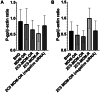
Combination therapy with small interfering RNA (siRNA) and chemotherapeutic drug is proven to be effective in downregulating cancer resistance proteins, such as P-glycoprotein (P-gp). These proteins are involved in multidrug resistance (MDR) of tumors. A targeted formulation capable of delivering siRNA and chemotherapeutic drug will not only downregulate P-gp but also increase the concentration of the chemotherapeutic drug at the site of tumor thereby increasing the therapeutic effect and lowering the systemic exposure. In this study, monoclonal antibody 2C5-modified dendrimer-based micelles were used to co-deliver siRNA and doxorubicin (DOX) to the tumor site in both male and female xenograft mouse model. The nucleosome-specific 2C5 antibody recognizes the cancer cells via the cell-surface bound nucleosomes. The ability of ability of the 2C5-modified formulation to affect the metastasis of highly aggressive triple negative breast cancer cell migration in (MDA-MB-231) was assessed by a wound healing. Further, the therapeutic efficacy of the formulation was assessed by measuring the tumor volume progression in which the 2C5-modified nanoparticle group had a similar tumor volume to the free drug group at the end of the study, although a 50% increase in DOX concentrations in blood was observed after the last dose of nanoparticle. The free drug group on the other hand showed body weight reduction as well as the visible irritation around the injection spot. The treatment group with 2C5-modified micelles has shown to be safe at the current dose of DOX and siRNA. Furthermore, the siRNA mediated P-gp downregualtion was studied using western blotting assay. We observed a 29% reduction of P-gp levels in both males and females with respect to the control (BHG). We also conclude that the dose of DOX and siRNA should be further optimized to have a better efficacy in a metastatic tumor model, which will be the subject of our future studies.
Keywords: Breast cancer; Co-delivery; Dendrimer; In vivo; Nanoparticles; Preclinical model; siRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Update of
-
Evaluation of mAb 2C5-modified dendrimer-based micelles for the co-delivery of siRNA and chemotherapeutic drug in xenograft mice model.Res Sq [Preprint]. 2023 Dec 11:rs.3.rs-3713164. doi: 10.21203/rs.3.rs-3713164/v1. Res Sq. 2023. Update in: Drug Deliv Transl Res. 2024 Aug;14(8):2171-2185. doi: 10.1007/s13346-024-01562-5. PMID: 38168301 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

