An organic O donor for biological hydroxylation reactions
- PMID: 38507448
- PMCID: PMC10990095
- DOI: 10.1073/pnas.2321242121
An organic O donor for biological hydroxylation reactions
Abstract
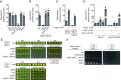
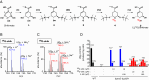
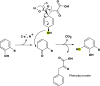
All biological hydroxylation reactions are thought to derive the oxygen atom from one of three inorganic oxygen donors, O2, H2O2, or H2O. Here, we have identified the organic compound prephenate as the oxygen donor for the three hydroxylation steps of the O2-independent biosynthetic pathway of ubiquinone, a widely distributed lipid coenzyme. Prephenate is an intermediate in the aromatic amino acid pathway and genetic experiments showed that it is essential for ubiquinone biosynthesis in Escherichia coli under anaerobic conditions. Metabolic labeling experiments with 18O-shikimate, a precursor of prephenate, demonstrated the incorporation of 18O atoms into ubiquinone. The role of specific iron-sulfur enzymes belonging to the widespread U32 protein family is discussed. Prephenate-dependent hydroxylation reactions represent a unique biochemical strategy for adaptation to anaerobic environments.
Keywords: U32 proteins; anaerobiosis; hydroxylation; prephenate; ubiquinone.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Torres Pazmiño D. E., Winkler M., Glieder A., Fraaije M. W., Monooxygenases as biocatalysts: Classification, mechanistic aspects and biotechnological applications. J. Biotechnol. 146, 9–24 (2010). - PubMed
-
- Hansen C. C., Nelson D. R., Møller B. L., Werck-Reichhart D., Plant cytochrome P450 plasticity and evolution. Mol. Plant 14, 1244–1265 (2021). - PubMed
-
- Paul C. E., Eggerichs D., Westphal A. H., Tischler D., van Berkel W. J. H., Flavoprotein monooxygenases: Versatile biocatalysts. Biotechnol. Adv. 51, 107712 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

