Reactive astrocytes secrete the chaperone HSPB1 to mediate neuroprotection
- PMID: 38507480
- PMCID: PMC10954207
- DOI: 10.1126/sciadv.adk9884
Reactive astrocytes secrete the chaperone HSPB1 to mediate neuroprotection
Abstract
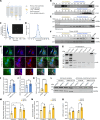
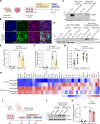
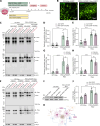
Molecular chaperones are protective in neurodegenerative diseases by preventing protein misfolding and aggregation, such as extracellular amyloid plaques and intracellular tau neurofibrillary tangles in Alzheimer's disease (AD). In addition, AD is characterized by an increase in astrocyte reactivity. The chaperone HSPB1 has been proposed as a marker for reactive astrocytes; however, its astrocytic functions in neurodegeneration remain to be elucidated. Here, we identify that HSPB1 is secreted from astrocytes to exert non-cell-autonomous protective functions. We show that in human AD brain, HSPB1 levels increase in astrocytes that cluster around amyloid plaques, as well as in the adjacent extracellular space. Moreover, in conditions that mimic an inflammatory reactive response, astrocytes increase HSPB1 secretion. Concomitantly, astrocytes and neurons can uptake astrocyte-secreted HSPB1, which is accompanied by an attenuation of the inflammatory response in reactive astrocytes and reduced pathological tau inclusions. Our findings highlight a protective mechanism in disease conditions that encompasses the secretion of a chaperone typically regarded as intracellular.
Figures





References
-
- Escartin C., Galea E., Lakatos A., O’Callaghan J. P., Petzold G. C., Serrano-Pozo A., Steinhäuser C., Volterra A., Carmignoto G., Agarwal A., Allen N. J., Araque A., Barbeito L., Barzilai A., Bergles D. E., Bonvento G., Butt A. M., Chen W.-T., Cohen-Salmon M., Cunningham C., Deneen B., De Strooper B., Díaz-Castro B., Farina C., Freeman M., Gallo V., Goldman J. E., Goldman S. A., Götz M., Gutiérrez A., Haydon P. G., Heiland D. H., Hol E. M., Holt M. G., Iino M., Kastanenka K. V., Kettenmann H., Khakh B. S., Koizumi S., Lee C. J., Liddelow S. A., MacVicar B. A., Magistretti P., Messing A., Mishra A., Molofsky A. V., Murai K. K., Norris C. M., Okada S., Oliet S. H. R., Oliveira J. F., Panatier A., Parpura V., Pekna M., Pekny M., Pellerin L., Perea G., Pérez-Nievas B. G., Pfrieger F. W., Poskanzer K. E., Quintana F. J., Ransohoff R. M., Riquelme-Perez M., Robel S., Rose C. R., Rothstein J. D., Rouach N., Rowitch D. H., Semyanov A., Sirko S., Sontheimer H., Swanson R. A., Vitorica J., Wanner I.-B., Wood L. B., Wu J., Zheng B., Zimmer E. R., Zorec R., Sofroniew M. V., Verkhratsky A., Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021). - PMC - PubMed
-
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., Bennett M. L., Münch A. E., Chung W.-S., Peterson T. C., Wilton D. K., Frouin A., Napier B. A., Panicker N., Kumar M., Buckwalter M. S., Rowitch D. H., Dawson V. L., Dawson T. M., Stevens B., Barres B. A., Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

