Adaptation in Unstable Environments and Global Gene Losses: Small but Stable Gene Networks by the May-Wigner Theory
- PMID: 38507653
- PMCID: PMC10991078
- DOI: 10.1093/molbev/msae059
Adaptation in Unstable Environments and Global Gene Losses: Small but Stable Gene Networks by the May-Wigner Theory
Abstract
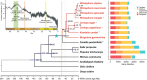
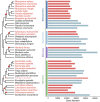
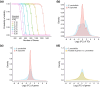
Although gene loss is common in evolution, it remains unclear whether it is an adaptive process. In a survey of seven major mangrove clades that are woody plants in the intertidal zones of daily environmental perturbations, we noticed that they generally evolved reduced gene numbers. We then focused on the largest clade of Rhizophoreae and observed the continual gene set reduction in each of the eight species. A great majority of gene losses are concentrated on environmental interaction processes, presumably to cope with the constant fluctuations in the tidal environments. Genes of the general processes for woody plants are largely retained. In particular, fewer gene losses are found in physiological traits such as viviparous seeds, high salinity, and high tannin content. Given the broad and continual genome reductions, we propose the May-Wigner theory (MWT) of system stability as a possible mechanism. In MWT, the most effective solution for buffering continual perturbations is to reduce the size of the system (or to weaken the total genic interactions). Mangroves are unique as immovable inhabitants of the compound environments in the land-sea interface, where environmental gradients (such as salinity) fluctuate constantly, often drastically. Extending MWT to gene regulatory network (GRN), computer simulations and transcriptome analyses support the stabilizing effects of smaller gene sets in mangroves vis-à-vis inland plants. In summary, we show the adaptive significance of gene losses in mangrove plants, including the specific role of promoting phenotype innovation and a general role in stabilizing GRN in unstable environments as predicted by MWT.
Keywords: adaptive evolution; gene loss; genome; mangrove; network stability; unstable environment.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Conflict of interest statement
None declared.
Figures



Similar articles
-
The origin, diversification and adaptation of a major mangrove clade (Rhizophoreae) revealed by whole-genome sequencing.Natl Sci Rev. 2017 Sep;4(5):721-734. doi: 10.1093/nsr/nwx065. Epub 2017 Jun 5. Natl Sci Rev. 2017. PMID: 31258950 Free PMC article.
-
Where whole-genome duplication is most beneficial: Adaptation of mangroves to a wide salinity range between land and sea.Mol Ecol. 2023 Jan;32(2):460-475. doi: 10.1111/mec.16320. Epub 2021 Dec 21. Mol Ecol. 2023. PMID: 34882881
-
Comparative Analysis of Transcriptomes in Rhizophoraceae Provides Insights into the Origin and Adaptive Evolution of Mangrove Plants in Intertidal Environments.Front Plant Sci. 2017 May 16;8:795. doi: 10.3389/fpls.2017.00795. eCollection 2017. Front Plant Sci. 2017. PMID: 28559911 Free PMC article.
-
Functional Traits of Terrestrial Plants in the Intertidal: A Review on Mangrove Trees.Biol Bull. 2021 Oct;241(2):123-139. doi: 10.1086/716510. Epub 2021 Oct 8. Biol Bull. 2021. PMID: 34706208 Review.
-
Convergent evolution of gene regulatory networks underlying plant adaptations to dry environments.Plant Cell Environ. 2021 Oct;44(10):3211-3222. doi: 10.1111/pce.14143. Epub 2021 Jul 12. Plant Cell Environ. 2021. PMID: 34196969 Free PMC article. Review.
Cited by
-
Genome-Wide Investigation of MADS-Box Genes in Flower Development and Environmental Acclimation of Lumnitzera littorea (Jack) Voigt.Int J Mol Sci. 2025 Feb 16;26(4):1680. doi: 10.3390/ijms26041680. Int J Mol Sci. 2025. PMID: 40004145 Free PMC article.
-
Conserved genome structure and phylogenetic insights for the heterogeneous subfamily of Convallarioideae (Asparagaceae) revealed from plastome data.BMC Plant Biol. 2025 May 27;25(1):710. doi: 10.1186/s12870-025-06711-7. BMC Plant Biol. 2025. PMID: 40426051 Free PMC article.
References
-
- Ball MC. Mangrove species richness in relation to salinity and waterlogging: a case study along the Adelaide River floodplain, northern Australia. Glob Ecol Biogeogr Lett. 1998:7(1):73–82. 10.2307/2997699. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

