The Diverse Evolutionary Histories of Domesticated Metaviral Capsid Genes in Mammals
- PMID: 38507667
- PMCID: PMC11011659
- DOI: 10.1093/molbev/msae061
The Diverse Evolutionary Histories of Domesticated Metaviral Capsid Genes in Mammals
Abstract
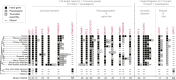
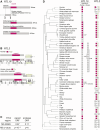
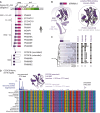
Selfish genetic elements comprise significant fractions of mammalian genomes. In rare instances, host genomes domesticate segments of these elements for function. Using a complete human genome assembly and 25 additional vertebrate genomes, we re-analyzed the evolutionary trajectories and functional potential of capsid (CA) genes domesticated from Metaviridae, a lineage of retrovirus-like retrotransposons. Our study expands on previous analyses to unearth several new insights about the evolutionary histories of these ancient genes. We find that at least five independent domestication events occurred from diverse Metaviridae, giving rise to three universally retained single-copy genes evolving under purifying selection and two gene families unique to placental mammals, with multiple members showing evidence of rapid evolution. In the SIRH/RTL family, we find diverse amino-terminal domains, widespread loss of protein-coding capacity in RTL10 despite its retention in several mammalian lineages, and differential utilization of an ancient programmed ribosomal frameshift in RTL3 between the domesticated CA and protease domains. Our analyses also reveal that most members of the PNMA family in mammalian genomes encode a conserved putative amino-terminal RNA-binding domain (RBD) both adjoining and independent from domesticated CA domains. Our analyses lead to a significant correction of previous annotations of the essential CCDC8 gene. We show that this putative RBD is also present in several extant Metaviridae, revealing a novel protein domain configuration in retrotransposons. Collectively, our study reveals the divergent outcomes of multiple domestication events from diverse Metaviridae in the common ancestor of placental mammals.
Keywords: LTR retrotransposon; PNMA; RNA-binding; SIRH; capsid; exaptation; gene conservation; positive selection.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures



Update of
-
The diverse evolutionary histories of domesticated metaviral capsid genes in mammals.bioRxiv [Preprint]. 2023 Sep 17:2023.09.17.558119. doi: 10.1101/2023.09.17.558119. bioRxiv. 2023. Update in: Mol Biol Evol. 2024 Apr 2;41(4):msae061. doi: 10.1093/molbev/msae061. PMID: 37745568 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

