A Compound That Inhibits Glycolysis in Prostate Cancer Controls Growth of Advanced Prostate Cancer
- PMID: 38507737
- PMCID: PMC11219269
- DOI: 10.1158/1535-7163.MCT-23-0540
A Compound That Inhibits Glycolysis in Prostate Cancer Controls Growth of Advanced Prostate Cancer
Abstract
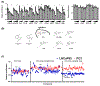
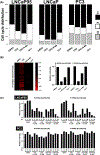
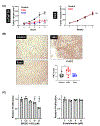
Metastatic castration-resistant prostate cancer remains incurable regardless of recent therapeutic advances. Prostate cancer tumors display highly glycolytic phenotypes as the cancer progresses. Nonspecific inhibitors of glycolysis have not been utilized successfully for chemotherapy, because of their penchant to cause systemic toxicity. This study reports the preclinical activity, safety, and pharmacokinetics of a novel small-molecule preclinical candidate, BKIDC-1553, with antiglycolytic activity. We tested a large battery of prostate cancer cell lines for inhibition of cell proliferation, in vitro. Cell-cycle, metabolic, and enzymatic assays were used to demonstrate their mechanism of action. A human patient-derived xenograft model implanted in mice and a human organoid were studied for sensitivity to our BKIDC preclinical candidate. A battery of pharmacokinetic experiments, absorption, distribution, metabolism, and excretion experiments, and in vitro and in vivo toxicology experiments were carried out to assess readiness for clinical trials. We demonstrate a new class of small-molecule inhibitors where antiglycolytic activity in prostate cancer cell lines is mediated through inhibition of hexokinase 2. These compounds display selective growth inhibition across multiple prostate cancer models. We describe a lead BKIDC-1553 that demonstrates promising activity in a preclinical xenograft model of advanced prostate cancer, equivalent to that of enzalutamide. BKIDC-1553 demonstrates safety and pharmacologic properties consistent with a compound that can be taken into human studies with expectations of a good safety margin and predicted dosing for efficacy. This work supports testing BKIDC-1553 and its derivatives in clinical trials for patients with advanced prostate cancer.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures



Update of
-
A Compound that Inhibits Glycolysis in Prostate Cancer Controls Growth of Advanced Prostate Cancer.bioRxiv [Preprint]. 2024 Feb 13:2023.07.01.547355. doi: 10.1101/2023.07.01.547355. bioRxiv. 2024. Update in: Mol Cancer Ther. 2024 Jul 2;23(7):973-994. doi: 10.1158/1535-7163.MCT-23-0540. PMID: 37461469 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

