Daratumumab monotherapy in refractory warm autoimmune hemolytic anemia and cold agglutinin disease
- PMID: 38507742
- PMCID: PMC11157213
- DOI: 10.1182/bloodadvances.2024012585
Daratumumab monotherapy in refractory warm autoimmune hemolytic anemia and cold agglutinin disease
Abstract
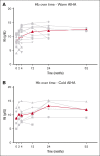
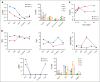
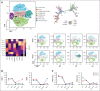
Autoimmune hemolytic anemia (AIHA) is a rare autoantibody-mediated disease. For steroid and/or rituximab-refractory AIHA, there is no consensus on optimal treatment. Daratumumab, a monoclonal antibody targeting CD38, could be beneficial by suppression of CD38+ plasma cells and thus autoantibody secretion. In addition, because CD38 is also expressed by activated T cells, daratumumab may also act via immunomodulatory effects. We evaluated the efficacy and safety of daratumumab monotherapy in an international retrospective study including 19 adult patients with heavily pretreated refractory AIHA. In warm AIHA (wAIHA, n = 12), overall response was 50% with a median response duration of 5.5 months (range, 2-12), including ongoing response in 2 patients after 6 and 12 months. Of 6 nonresponders, 4 had Evans syndrome. In cold AIHA (cAIHA, n = 7) overall hemoglobin (Hb) response was 57%, with ongoing response in 3 of 7 patients. One additional patient with nonanemic cAIHA was treated for severe acrocyanosis and reached a clinical acrocyanosis response as well as a Hb increase. Of 6 patients with cAIHA with acrocyanosis, 4 had improved symptoms after daratumumab treatment. In 2 patients with wAIHA treated with daratumumab, in whom we prospectively collected blood samples, we found complete CD38+ T-cell depletion after daratumumab, as well as altered T-cell subset differentiation and a severely diminished capacity for cell activation and proliferation. Reappearance of CD38+ T cells coincided with disease relapse in 1 patient. In conclusion, our data show that daratumumab therapy may be a treatment option for refractory AIHA. The observed immunomodulatory effects that may contribute to the clinical response deserve further exploration.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: B.F. received consultancy honoraria from Alexion, Novartis, Janssen, and Sobi. M.M. received consultancy fees and/or speakers fees received from Novartis, Alexion, Sanofi, Union Chimique Belge, Argenx, and Sobi. E.C. received honoraria (advisory boards, speaker’s fees) from Novartis, Union Chimique Belge, Amgen, and Sanofi. Q.A.H. received speaker honoraria from Grifols and Novartis; consultancy from Amgen, Argenx, Gliknik, Incyte, Immunovant, Janssen, Novartis, Sanofi, and Sobi. U.J. received honoraria from Sanofi, Roche, Novartis, Incyte, Janssen, and Bristol Myers Squibb; advisory role fees from Sanofi, Roche, and Novartis. A.K. received research funding from AbbVie, AstraZeneca, Bristol Myers Squibb, Janssen, and Roche/Genentech; received patent royalties from Janssen and LAVA; and served on the board of directors or advisory committees for AstraZeneca, BMS, Roche/Genentech, Janssen, AbbVie, and LAVA; and received speaker’s fees from AbbVie, AstraZeneca, and Janssen. S.D.S. received research funding from BeiGene and Janssen; advisory board fees from BeiGene, Sanofi, Janssen and Cellectar; speakers bureau fees from Janssen and BeiGene. J.M.I.V. received consultancy and advisory board honoraria from Sanofi and Janssen; research support from BeiGene and AbbVie/Genmab; and participated in the speakers’ bureau for Bristol Myers Squibb, Sanofi, and Amgen. All honoraria received are directed to the institute. The remaining authors declare no competing financial interests.
Figures
References
-
- Jager U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020;41 - PubMed
-
- Berentsen S, Barcellini W, D'Sa S, et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood. 2020;136(4):480–488. - PubMed
-
- Berentsen S, D’Sa S, Randen U, Małecka A, Vos JMI. Cold agglutinin disease: improved understanding of pathogenesis helps define targets for therapy. Hemato. 2022;3(4):574–594.
-
- Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4):460–466. - PubMed
-
- Berentsen S, Barcellini W, D'Sa S, Jilma B. Sutimlimab for treatment of cold agglutinin disease: why, how and for whom? Immunotherapy. 2022;14(15):1191–1204. - PubMed

