Risk of Autism after Prenatal Topiramate, Valproate, or Lamotrigine Exposure
- PMID: 38507750
- PMCID: PMC11047762
- DOI: 10.1056/NEJMoa2309359
Risk of Autism after Prenatal Topiramate, Valproate, or Lamotrigine Exposure
Abstract
Background: Maternal use of valproate during pregnancy has been associated with an increased risk of neurodevelopmental disorders in children. Although most studies of other antiseizure medications have not shown increased risks of these disorders, there are limited and conflicting data regarding the risk of autism spectrum disorder associated with maternal topiramate use.
Methods: We identified a population-based cohort of pregnant women and their children within two health care utilization databases in the United States, with data from 2000 through 2020. Exposure to specific antiseizure medications was defined on the basis of prescription fills from gestational week 19 until delivery. Children who had been exposed to topiramate during the second half of pregnancy were compared with those unexposed to any antiseizure medication during pregnancy with respect to the risk of autism spectrum disorder. Valproate was used as a positive control, and lamotrigine was used as a negative control.
Results: The estimated cumulative incidence of autism spectrum disorder at 8 years of age was 1.9% for the full population of children who had not been exposed to antiseizure medication (4,199,796 children). With restriction to children born to mothers with epilepsy, the incidence was 4.2% with no exposure to antiseizure medication (8815 children), 6.2% with exposure to topiramate (1030 children), 10.5% with exposure to valproate (800 children), and 4.1% with exposure to lamotrigine (4205 children). Propensity score-adjusted hazard ratios in a comparison with no exposure to antiseizure medication were 0.96 (95% confidence interval [CI], 0.56 to 1.65) for exposure to topiramate, 2.67 (95% CI, 1.69 to 4.20) for exposure to valproate, and 1.00 (95% CI, 0.69 to 1.46) for exposure to lamotrigine.
Conclusions: The incidence of autism spectrum disorder was higher among children prenatally exposed to the studied antiseizure medications than in the general population. However, after adjustment for indication and other confounders, the association was substantially attenuated for topiramate and lamotrigine, whereas an increased risk remained for valproate. (Funded by the National Institute of Mental Health.).
Copyright © 2024 Massachusetts Medical Society.
Figures

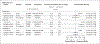

References
-
- Tomson T, Battino D, Bromley R, et al. Executive summary: management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epilepsia 2019;60:2343–5. - PubMed
-
- Tomson T, Battino D, Perucca E. Teratogenicity of antiepileptic drugs. Curr Opin Neurol 2019;32:246–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
