Co-immobilization of Ciprofloxacin and Chlorhexidine as a Broad-Spectrum Antimicrobial Dual-Drug Coating for Poly(vinyl chloride) (PVC)-Based Endotracheal Tubes
- PMID: 38507790
- PMCID: PMC10995906
- DOI: 10.1021/acsami.4c01334
Co-immobilization of Ciprofloxacin and Chlorhexidine as a Broad-Spectrum Antimicrobial Dual-Drug Coating for Poly(vinyl chloride) (PVC)-Based Endotracheal Tubes
Erratum in
-
Correction to "Co-immobilization of Ciprofloxacin and Chlorhexidine as a Broad-Spectrum Antimicrobial Dual-Drug Coating for Poly(vinyl chloride) (PVC)-Based Endotracheal Tubes".ACS Appl Mater Interfaces. 2024 Jul 3;16(26):34433-34434. doi: 10.1021/acsami.4c09227. Epub 2024 Jun 18. ACS Appl Mater Interfaces. 2024. PMID: 38961581 Free PMC article. No abstract available.
Abstract
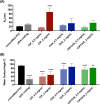
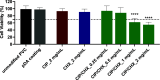
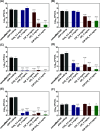
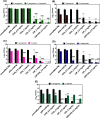
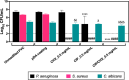
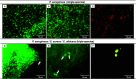
The endotracheal tube (ETT) affords support for intubated patients, but the increasing incidence of ventilator-associated pneumonia (VAP) is jeopardizing its application. ETT surfaces promote (poly)microbial colonization and biofilm formation, with a heavy burden for VAP. Devising safe, broad-spectrum antimicrobial materials to tackle the ETT bioburden is needful. Herein, we immobilized ciprofloxacin (CIP) and/or chlorhexidine (CHX), through polydopamine (pDA)-based functionalization, onto poly(vinyl chloride) (PVC) surfaces. These surfaces were characterized regarding physicochemical properties and challenged with single and polymicrobial cultures of VAP-relevant bacteria (Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, Staphylococcus aureus, Staphylococcus epidermidis) and fungi (Candida albicans). The coatings imparted PVC surfaces with a homogeneous morphology, varied wettability, and low roughness. The antimicrobial immobilization via pDA chemistry was still evidenced by infrared spectroscopy. Coated surfaces exhibited sustained CIP/CHX release, retaining prolonged (10 days) activity. CIP/CHX-coated surfaces evidencing no A549 lung cell toxicity displayed better antibiofilm outcomes than CIP or CHX coatings, preventing bacterial attachment by 4.1-7.2 Log10 CFU/mL and modestly distressingC. albicans. Their antibiofilm effectiveness was endured toward polymicrobial consortia, substantially inhibiting the adhesion of the bacterial populations (up to 8 Log10 CFU/mL) within the consortia in dual- and even inP. aeruginosa/S. aureus/C. albicans triple-species biofilms while affecting fungal adhesion by 2.7 Log10 CFU/mL (dual consortia) and 1 Log10 CFU/mL (triple consortia). The potential of the dual-drug coating strategy in preventing triple-species adhesion and impairing bacterial viability was still strengthened by live/dead microscopy. The pDA-assisted CIP/CHX co-immobilization holds a safe and robust broad-spectrum antimicrobial coating strategy for PVC-ETTs, with the promise laying in reducing VAP incidence.
Keywords: co-immobilization; dual-drug coating; endotracheal tube; polymicrobial biofilm; ventilator-associated pneumonia.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Ippolito M.; Misseri G.; Catalisano G.; Marino C.; Ingoglia G.; Alessi M.; Consiglio E.; Gregoretti C.; Giarratano A.; Cortegiani A. Ventilator-Associated Pneumonia in Patients with Covid-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10 (5), 54510.3390/antibiotics10050545. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

