GAA-FGF14 disease: defining its frequency, molecular basis, and 4-aminopyridine response in a large downbeat nystagmus cohort
- PMID: 38507876
- PMCID: PMC10960126
- DOI: 10.1016/j.ebiom.2024.105076
GAA-FGF14 disease: defining its frequency, molecular basis, and 4-aminopyridine response in a large downbeat nystagmus cohort
Abstract
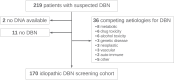
Background: GAA-FGF14 disease/spinocerebellar ataxia 27B is a recently described neurodegenerative disease caused by (GAA)≥250 expansions in the fibroblast growth factor 14 (FGF14) gene, but its phenotypic spectrum, pathogenic threshold, and evidence-based treatability remain to be established. We report on the frequency of FGF14 (GAA)≥250 and (GAA)200-249 expansions in a large cohort of patients with idiopathic downbeat nystagmus (DBN) and their response to 4-aminopyridine.
Methods: Retrospective cohort study of 170 patients with idiopathic DBN, comprising in-depth phenotyping and assessment of 4-aminopyridine treatment response, including re-analysis of placebo-controlled video-oculography treatment response data from a previous randomised double-blind 4-aminopyridine trial.
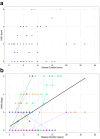
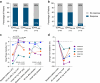
Findings: Frequency of FGF14 (GAA)≥250 expansions was 48% (82/170) in patients with idiopathic DBN. Additional cerebellar ocular motor signs were observed in 100% (82/82) and cerebellar ataxia in 43% (35/82) of patients carrying an FGF14 (GAA)≥250 expansion. FGF14 (GAA)200-249 alleles were enriched in patients with DBN (12%; 20/170) compared to controls (0.87%; 19/2191; OR, 15.20; 95% CI, 7.52-30.80; p < 0.0001). The phenotype of patients carrying a (GAA)200-249 allele closely mirrored that of patients carrying a (GAA)≥250 allele. Patients carrying a (GAA)≥250 or a (GAA)200-249 allele had a significantly greater clinician-reported (80%, 33/41 vs 31%, 5/16; RR, 2.58; 95% CI, 1.23-5.41; Fisher's exact test, p = 0.0011) and self-reported (59%, 32/54 vs 11%, 2/19; RR, 5.63; 95% CI, 1.49-21.27; Fisher's exact test, p = 0.00033) response to 4-aminopyridine treatment compared to patients carrying a (GAA)<200 allele. Placebo-controlled video-oculography data, available for four patients carrying an FGF14 (GAA)≥250 expansion, showed a significant decrease in slow phase velocity of DBN with 4-aminopyridine, but not placebo.
Interpretation: This study confirms that FGF14 GAA expansions are a frequent cause of DBN syndromes. It provides preliminary evidence that (GAA)200-249 alleles might be pathogenic. Finally, it provides large real-world and preliminary piloting placebo-controlled evidence for the efficacy of 4-aminopyridine in GAA-FGF14 disease.
Funding: This work was supported by the Clinician Scientist program "PRECISE.net" funded by the Else Kröner-Fresenius-Stiftung (to CW, AT, and MSy), the grant 779257 "Solve-RD" from the European's Union Horizon 2020 research and innovation program (to MSy), and the grant 01EO 1401 by the German Federal Ministry of Education and Research (BMBF) (to MSt). This work was also supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) N° 441409627, as part of the PROSPAX consortium under the frame of EJP RD, the European Joint Programme on Rare Diseases, under the EJP RD COFUND-EJP N° 825575 (to MSy, BB and-as associated partner-SZ), the NIH National Institute of Neurological Disorders and Stroke (grant 2R01NS072248-11A1 to SZ), the Fondation Groupe Monaco (to BB), and the Montreal General Hospital Foundation (grant PT79418 to BB). The Care4Rare Canada Consortium is funded in part by Genome Canada and the Ontario Genomics Institute (OGI-147 to KMB), the Canadian Institutes of Health Research (CIHR GP1-155867 to KMB), Ontario Research Foundation, Genome Quebec, and the Children's Hospital of Eastern Ontario Foundation. The funders had no role in the conduct of this study.
Keywords: 4-Aminopyridine; Downbeat nystagmus; GAA-FGF14 ataxia; SCA27B; Treatment; Trial.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests DP, FH, CW, MCD, AT, CA, MJD, AC, GDG, KMB, JC, AMH, and BB report no disclosures. DR has received grant/research support from Janssen and Lundbeck; he has served as a consultant or on advisory boards for AC Immune, Janssen, Roche and Rovi and he has served on speakers bureaus of Janssen and Pharmagenetix. He also received honoraria from Gerot Lannacher, Janssen and Pharmagenetix, and travel support from Angelini and Janssen, all unrelated to the present manuscript. SZ has received consultancy honoraria from Neurogene, Aeglea BioTherapeutics, Applied Therapeutics, and is an unpaid officer of the TGP foundation, all unrelated to the present manuscript. MSt is Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro-otology and Section Editor of F1000. He has received speakers honoraria from Abbott, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, J&J, MSD, NeuroUpdate, Otometrics, Pierre-Fabre, TEVA, UCB, and Viatris. He receives support for clinical studies from Decibel, U.S.A., Cure within Reach, U.S.A. and Heel, Germany. He distributes M-glasses and Positional vertigo App. He acts as a consultant for Abbott, AurisMedical, Bulbitec, Heel, IntraBio, Sensorion and Vertify. He is an investor and share-holder of IntraBio. All are unrelated to the present manuscript. MSy has received consultancy honoraria from Janssen, Ionis, Orphazyme, Servier, Reata, Biohaven, Zevra, Lilly, GenOrph, and AviadoBio, all unrelated to the present manuscript. MSy is planning a treatment trial of 4-AP in GAA-FGF14 disease together with Solaxa Inc. as a sponsor, but has not received any type of honoraria or funding from Solaxa.
Figures




Update of
-
Intronic FGF14 GAA repeat expansions are a common cause of downbeat nystagmus syndromes: frequency, phenotypic profile, and 4-aminopyridine treatment response.medRxiv [Preprint]. 2023 Aug 5:2023.07.30.23293380. doi: 10.1101/2023.07.30.23293380. medRxiv. 2023. Update in: EBioMedicine. 2024 Apr;102:105076. doi: 10.1016/j.ebiom.2024.105076. PMID: 37577458 Free PMC article. Updated. Preprint.
References
-
- Wilke C., Pellerin D., Mengel D., et al. GAA-FGF14 ataxia (SCA27B): phenotypic profile, natural history progression and 4-aminopyridine treatment response. Brain. 2023;146(10):4144–4157. - PubMed
-
- Pellerin D., Danzi M., Renaud M., et al. In: GeneReviews(®) Adam M.P., Feldman J., Mirzaa G.M., et al., editors. 2024. GAA-FGF14-Related ataxia. Seattle (WA): University of Washington, Seattle.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

