Hepatotoxicity in patients with non-small cell lung cancer treated with sotorasib after prior immunotherapy: a comprehensive clinical and pharmacokinetic analysis
- PMID: 38507877
- PMCID: PMC10960098
- DOI: 10.1016/j.ebiom.2024.105074
Hepatotoxicity in patients with non-small cell lung cancer treated with sotorasib after prior immunotherapy: a comprehensive clinical and pharmacokinetic analysis
Abstract
Background: Sotorasib given after immunotherapy could put patients at increased risk of hepatotoxicity. Therefore, there is a need to gain insight into the potential correlation between anti-PD-(L)1 treatment, anti-PD-(L)1 concentrations, sotorasib concentrations, and the incidence of hepatotoxicity during sotorasib.
Methods: Patients with KRASG12C-mutated NSCLC treated with sotorasib were prospectively enrolled in our biomarker cohort study (NCT05221372). Plasma samples were collected prior and during sotorasib treatment for anti-PD-1 and sotorasib concentrations. ALT/AST/ALP/GGT increases were collected prospectively and graded according to CTCAEv5.0. Severe hepatotoxicity was defined as grade ≥3 ALT/AST/ALP/GGT increase.
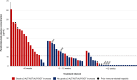
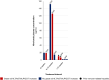
Findings: Of the 91 included patients, 80 (88%) received prior anti-PD-(L)1. Prior anti-PD-(L)1 and prior immune-related hepatotoxicity were associated with a higher incidence of severe hepatotoxicity (35% versus 0%, p = 0.016 and 75% versus 31%, p = 0.019, respectively). Patients with an interval of ≤6 weeks between anti-PD-(L)1 and sotorasib (n = 18) had a significantly higher incidence of severe hepatotoxicity than those with a 6-12 week (n = 24) and ≥12 week (n = 38) interval (83% versus 33% versus 13%, respectively, p < 0.0001). Sotorasib trough concentrations did not differ significantly between those with or without severe hepatotoxicity (106 versus 126 ng/mL, p = 0.16). Pembrolizumab concentrations were higher in those with severe hepatotoxicity versus those without (25.6 versus 6.1 μg/mL, p < 0.0001).
Interpretation: In this preliminary prospective study, sotorasib after PD-(L)1 blockade was associated with severe hepatotoxicity, especially in patients with a short interval between treatments, prior immune-related hepatitis and higher anti-PD-1 plasma concentrations. Our results suggest a minimum interval of 6 weeks between anti-PD-(L)1 and sotorasib to minimize the risk of hepatotoxicity.
Funding: None.
Keywords: Hepatitis; Immunotherapy; KRAS; NSCLC; Sotorasib.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests M.S. Paats reports receiving institutional fees from AstraZeneca, Bayer, Eli Lilly, Janssen, Novartis, Pfizer, Roche and Takeda; outside the current work. J.G.J.V. Aerts reports receiving advisory board and speakers fees from Eli Lilly, BMS, MSD, AstraZeneca, Bayer, Amphera and is a stock owner of Amphera; outside the current work. D.W. Dumoulin reports receiving consulting fees from BMS, MSD, Pfizer, Amgen and Roche; outside the current work. R.C. Cornelissen reports receiving advisory board and speakers fees from MSD, Janssen, Librerium and Spectrum; outside the current work. J.H. von der Thüsen reports receiving advisory board and speakers fees from Eli Lilly, BMS, MSD, AstraZeneca, Bayer, Janssen, Pfizer, Amgen, and institutional receipt of materials of Roche; outside the current work. R.H.J. Mathijssen reports receiving institutional fees for investigator-initiated trials from Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Novartis, Pamgene, Pfizer, Roche, Sanofi and Servier; outside the current work. S.L.W. Koolen reports receiving speakers fee from Promise Proteomics; outside the current work. A.C Dingemans reports receiving institutional fees from Roche, Eli Lilly, Boehringer Ingelheim, AstraZeneca, Janssen, Chiezi, Amgen, Pfizer, Bayer, Takeda, Pharmamar, Sanofi and Daiichi, and grants from the Dutch Cancer Society, HANARTH and Amgen paid to the institution; outside the current work; chair EORTC Lung Cancer group. All other authors report no disclosures.
Figures
References
-
- Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. - PubMed
-
- Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. - PubMed
-
- de Langen A.J., Johnson M.L., Mazieres J., et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401(10378):733–746. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

