Growth Trajectories Over the First Year of Life Among Early-Treated Infants with Human Immunodeficiency Virus and Infants Who are Human Immunodeficiency Virus-Exposed Uninfected
- PMID: 38508485
- PMCID: PMC11176027
- DOI: 10.1016/j.jpeds.2024.114018
Growth Trajectories Over the First Year of Life Among Early-Treated Infants with Human Immunodeficiency Virus and Infants Who are Human Immunodeficiency Virus-Exposed Uninfected
Abstract
Objective: To investigate the role of early antiretroviral therapy (ART) on growth trajectories of infants with human immunodeficiency virus (IHIV) in the first year of life.
Study design: As part of a clinical trial of early ART in Johannesburg, South Africa (2015-2018), 116 IHIV diagnosed within 48 hours of birth were started on ART as soon as possible, and 80 uninfected infants born to mothers living with HIV (IHEU) were enrolled. Both groups were followed prospectively from birth through 48 weeks and growth parameters collected. The groups were compared and risk factors for poor growth investigated, in the full cohort and among IHIV separately.
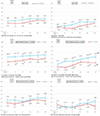
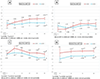
Results: IHIV had lower mean weight-for-age Z-scores (WAZ) than IHEU at 4 and 8 weeks (-1.17 [SE:0.14] vs -0.72 [0.14], P = .035 and -1.23 [0.15] vs -0.67 [0.14], P = .012). Although there was some closing of the gap over time, means remained lower in IHIV through 48 weeks. In length-for-age Z-scores (LAZ), differences widened over time and IHIV had lower Z-scores by 48 weeks (-1.41 [0.15] vs -0.80 [0.18], P = .011). Deficits in WAZ and LAZ in IHIV vs IHEU were most marked among girls. IHIV with pre-ART viral load ≥1000 copies/ml had significantly lower weight-for-length and mid-upper arm circumference Z-scores across all time points through 48 weeks.
Conclusions: IHIV on early ART had deficits in WAZ over the first 8 weeks of life and lower LAZ at 48 weeks than IHEU. Among IHIV, higher pre-ART viral load was associated with worse anthropometric indicators through 48 weeks.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Funding: The Latency and Early Neonatal Provision of Antiretroviral Drugs (LEOPARD) study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health (U01HD080441), the South African National HIV Programme, and South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa (84177).
Figures

References
-
- Flynn PM, Taha TE, Cababasay M, Fowler MG, Mofenson LM, Owor M, et al. Prevention of HIV-1 Transmission Through Breastfeeding: Efficacy and Safety of Maternal Antiretroviral Therapy Versus Infant Nevirapine Prophylaxis for Duration of Breastfeeding in HIV-1-Infected Women With High CD4 Cell Count (IMPAACT PROMISE): A Randomi. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2018;77:383–92. - PMC - PubMed
-
- Sibiude J, Le Chenadec J, Mandelbrot L, Hoctin A, Dollfus C, Faye A, et al. Update of Perinatal Human Immunodeficiency Virus Type 1 Transmission in France: Zero Transmission for 5482 Mothers on Continuous Antiretroviral Therapy From Conception and With Undetectable Viral Load at Delivery. Clinical Infectious Diseases. 2023;76:e590–e8. - PubMed
-
- Shiau S, Arpadi S, Strehlau R, Martens L, Patel F, Coovadia A, et al. Initiation of Antiretroviral Therapy Before 6 Months of Age is Associated with Faster Growth Recovery in South African Children Perinatally Infected with Human Immunodeficiency Virus. The Journal of Pediatrics. 2013;162:1138–45.e2. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

