Pediatric Consent on FHIR
- PMID: 38508581
- PMCID: PMC11078568
- DOI: 10.1055/a-2291-1482
Pediatric Consent on FHIR
Abstract
Background: Standardizing and formalizing consent processes and forms can prevent ambiguities, convey a more precise meaning, and support machine interpretation of consent terms.
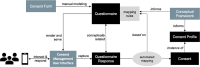
Objectives: Our goal was to introduce a systematic approach to standardizing and digitizing pediatric consent forms, which are complex due to legal requirements for child and legal guardian involvement.
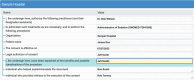
Methods: First, we reviewed the consent requirements from the Arizona regulation, and we used 21 pediatric treatment consents from five Arizona health care organizations to propose and evaluate an implementation-agnostic Consent for Treatment Framework. Second, we assessed the adequacy of the Fast Healthcare Interoperability Resources (FHIR) to support the proposed framework.
Results: The resulting Consent for Treatment Framework supports compliance with the state consent requirements and has been validated with pediatric consent forms. We also demonstrated that the FHIR standard has the required expressiveness to compute the framework's specifications and express the 21 consent forms.
Conclusion: Health care organizations can apply the shared open-source code and FHIR implementation guidelines to standardize the design of machine-interpretable pediatric treatment consent forms. The resulting FHIR-based executable models may support compliance with the law and support interoperability and data sharing.
Thieme. All rights reserved.
Conflict of interest statement
None declared.
Figures








References
-
- Murray B. Informed consent: what must a physician disclose to a patient? Virtual Mentor. 2012;14(07):563–566. - PubMed
-
- Shah P, Thornton I, Turrin D, Hipskind J E. Treasure Island (FL): StatPearls Publishing; 2023. Informed Consent. - PubMed
-
- Curcin V, Miles S, Danger R, Chen Y, Bache R, Taweel A. Implementing interoperable provenance in biomedical research. Future Gener Comput Syst. 2014;34:1–16.
-
- Zutlevics T L, Henning P H. Obligation of clinicians to treat unwilling children and young people: an ethical discussion. J Paediatr Child Health. 2005;41(12):677–681. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

